Atrioventricular (AV) block and endocardial fibroelastosis associated with dilated cardiomyopathy are the most common clinical manifestations of anti-Ro/SSA-mediated fetal cardiac disease. Valvar dysfunction has not been a prominent feature of this disease; however, recent anecdotal cases have suggested an association between rupture of the AV valve tensor apparatus and maternal anti-Ro/SSA antibodies. In the present study, we have described the clinical and laboratory findings and reviewed the published data for infants of anti-Ro/SSA-positive pregnancies with AV valve insufficiency due to chordal rupture from the papillary muscles. The histopathologic features of the papillary muscle and ventricular free wall and septum biopsy specimens were examined and compared to the sections of AV leaflets from 6 autopsied fetuses with anti-Ro/SSA-mediated complete AV block without chordal disruption. Specific epitopes to the p200 region of Ro52, and Ro60 antibodies were evaluated in cases with chordal rupture. Severe AV valve insufficiency was detected prenatally (as early as 34 weeks of gestation) or postnatally (as late as 182 days) after areas of patchy echogenicity were noted in the papillary muscle at 19 to 22 weeks of gestation. Postnatally, urgent valve surgery was performed in 5 of 6 patients; 1 of 6 patients died preoperatively. All patients tested positive for Ro52. Valve leaflet tissue from the autopsy specimens was normal. The ventricular free wall and septum biopsy specimens from a patient with chordal rupture showed normal tissue; however, the papillary muscle biopsy specimens demonstrated severe atrophy with near total replacement of myocytes by fibrosis and dystrophic calcifications, and negative immunochemistry findings. In conclusion, these findings have defined an underappreciated complication of fetal antibody-mediated cardiac inflammation.
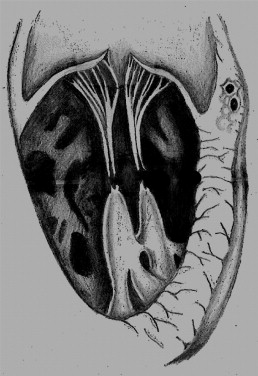
The spectrum of maternal anti-Ro/SSA antibody-mediated fetal cardiac disease includes conduction system disease primarily involving, but not limited to, the atrioventricular (AV) node, and myocardial disease in the form of endocardial fibroelastosis and dilated cardiomyopathy. Prenatal echocardiographic signs of fibrosis will manifest as areas of patchy echogenicity on the endocardial surfaces of the fetal heart. These often resolve but occasionally progress to endocardial fibroelastosis, ventricular dysfunction, and dilated cardiomyopathy. Although the papillary muscles and chordae of the AV valves are common sites of patchy echogenicity, severe AV-valvar insufficiency due to dysfunction of the tensor apparatus has rarely been reported. Recently, we cared for 2 infants from anti-Ro/SSA-positive pregnancies with fetal endocardial fibroelastosis and spontaneous disruption of the tensor apparatus of the AV valve ( Figure 1 ), resulting in sudden-onset, severe AV valve insufficiency. Four similar cases have been previously reported. The purpose of the present report was to summarize the clinical findings from all 6 cases and to provide new pathologic-immunologic insights into this unusual manifestation of immune-mediated cardiac disease. We speculated that the spontaneous disruption of the AV valve tensor apparatus might become more common as the survival of affected fetuses from anti-Ro/SSA-positive pregnancies continues to improve.
Case Reports
The 4 previously reported patients have been designated patients 3 to 6 and the new patients as patients 1 and 2.
Patient 1
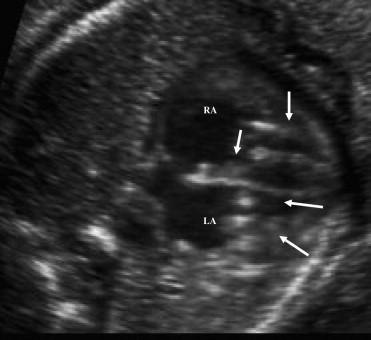
A 27-year-old anti-Ro/SSA-positive secundigravida mother was referred because of fetal bradycardia at 21 weeks of gestation. The fetal echocardiogram revealed a 3° AV block and focal areas of increased echogenicity in the papillary muscles of both AV valves and in the left atrium ( Figure 2 ). The mother was given oral dexamethasone (8 mg/day for 2 weeks, 4 mg/day from 23 to 30 weeks, and 2 mg/day until delivery) and intravenous immunoglobulin (70 g every 2 weeks to birth), with no change in the patchy echogenicity of the papillary muscles.
A 2.2 kg infant was delivered electively at term (38 weeks of gestation). The infant received 1 dose of intravenous immunoglobulin (2 g/kg) after delivery and oral prednisone for 2 months. The postnatal echocardiograms at birth and at 6 weeks showed mild residual patchy echogenicity of the tricuspid valve papillary muscles but no tricuspid valve insufficiency.
At 6 months of age, the infant developed cyanosis and respiratory distress. The preoperative echocardiogram revealed severe tricuspid insufficiency due to a flailed anterior tricuspid valve leaflet. The intraoperative findings included disruption of the chordal attachments of the anterior leaflet from the head of the papillary muscle. The chordae of the posterior leaflet appeared brittle and fibrotic; the chordae of the septal leaflet appeared unaffected. The disrupted chordae were replaced by 5-0 Gore-Tex, and back-up chordae were placed to augment the posterior leaflet. Biopsy specimens from the ventricular septum and papillary muscles were obtained. The child did well and, at age 3 years, had normal biventricular function, mild tricuspid insufficiency, and an echogenic tricuspid tensor apparatus.
Patient 2
A secundigravida anti-Ro/SSA-positive 25-year-old woman presented at 19 weeks of gestation with fetal tachycardia. The fetal echocardiogram showed a structurally normal heart, a small pericardial effusion, echogenic tricuspid valve papillary muscles, and nonsinus tachycardia with 1:1 AV association and a rate of 205 beats/min. The biventricular function was subjectively normal. During the next few weeks, the tachycardia resolved.
At 34 weeks of gestation, tricuspid valve insufficiency with dilation of the right atrium and right ventricle was noted. Despite treatment with transplacental dexamethasone 4 mg/day and one dose of intravenous immunoglobulin (1 g/kg), the insufficiency rapidly worsened, prompting delivery at 36 weeks of gestation. The infant weighed 2.8 kg. The postnatal echocardiogram showed normal biventricular function, a flailed anterior tricuspid valve leaflet with free tricuspid insufficiency, and mild pulmonary insufficiency. At 23 days, the anterior leaflet of the mitral valve became flail with severe mitral insufficiency. The infant developed pulmonary hemorrhage and renal failure and died at 32 days of age. A consent for autopsy was refused.
Methods
Intraoperative biopsies of the papillary muscles, ventricular septum, and anterior wall of the right ventricle were immediately fixed in buffered formalin for 24 hours. The tissues were embedded in paraffin, and 5-μm tissue sections were prepared for microscopy and histochemical staining, including hematoxylin-eosin staining, periodic acid-Schiff staining for the demonstration of tissue glycogen, and Movat pentachrome staining for the demonstration of elastin fibers, tissue protein, and collagen. Immunostain procedures were performed using an automated immunostainer (Benchmark, Ventana/Roche Diagnostics, Tucson, Arizona). Antigen retrieval procedures were applied to the dewaxed and rehydrated tissue slides. Biotinylated antibodies to cleaved caspase 3 were used at a dilution of 1/200.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) analysis was performed on the papillary muscle tissue. In situ hybridization was performed using an automated stainer (Discovery model, Ventana/Roche Diagnostics). The slides were incubated with probe and recombinant terminal deoxynucleotidyl enzyme (Gibco, Carlsbad, California), (Roche, Basel, Switzerland), and the in situ hybridization reaction performed under conditions preset by the Autostainer program. The tissue slides were counter stained with hematoxylin.
From the archive at the Royal Brompton Hospital, we reviewed the histologic features of the mitral and tricuspid valve leaflets of 6 fetal hearts with maternal anti-Ro/SSA antibodies. These cases had complete AV block without disruption of the tensor apparatus of the AV valves. The tissue blocks had been serially cut at 10 μm, and sections at 250-μm intervals were stained with Masson’s trichrome. From the remainder, we retrieved representative sections of the AV valves and stained them with elastic-Van Gieson stain for examination of the leaflet architecture. These cases were examined for comparison with patient 1.
We obtained maternal serum from patients 1 and 2 and the previously published patients 3 and 4 for Ro52, Ro60, and La antibody testing. Using the INNO-LIA ANA Update (Innogenetics, Ghent, Belgium) line immunoblot assay according to the manufacturer’s instructions, Ro52 antibodies and antibodies to a specific epitope encoded within amino acid 200-239 (p200) of Ro52 were quantified using enzyme-linked immunosorbent assay with recombinant, bacterially expressed, and purified Ro52 antigen encoded in the pMAL vector system and a synthetized p200 peptide, as previously described. The sera were tested at a dilution of 1:500, and bound antibodies were detected by affinity-purified alkaline phosphatase-conjugated anti-IgG antibodies using p-nitrophenyl phosphate disodium salt as a substrate and registration of the optical density at 405 nm. Sera with high and low Ro52 and p200 antibody levels and mouse anti-human Ro52 monoclonal antibodies generated by us were used as internal controls.
Methods
Intraoperative biopsies of the papillary muscles, ventricular septum, and anterior wall of the right ventricle were immediately fixed in buffered formalin for 24 hours. The tissues were embedded in paraffin, and 5-μm tissue sections were prepared for microscopy and histochemical staining, including hematoxylin-eosin staining, periodic acid-Schiff staining for the demonstration of tissue glycogen, and Movat pentachrome staining for the demonstration of elastin fibers, tissue protein, and collagen. Immunostain procedures were performed using an automated immunostainer (Benchmark, Ventana/Roche Diagnostics, Tucson, Arizona). Antigen retrieval procedures were applied to the dewaxed and rehydrated tissue slides. Biotinylated antibodies to cleaved caspase 3 were used at a dilution of 1/200.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) analysis was performed on the papillary muscle tissue. In situ hybridization was performed using an automated stainer (Discovery model, Ventana/Roche Diagnostics). The slides were incubated with probe and recombinant terminal deoxynucleotidyl enzyme (Gibco, Carlsbad, California), (Roche, Basel, Switzerland), and the in situ hybridization reaction performed under conditions preset by the Autostainer program. The tissue slides were counter stained with hematoxylin.
From the archive at the Royal Brompton Hospital, we reviewed the histologic features of the mitral and tricuspid valve leaflets of 6 fetal hearts with maternal anti-Ro/SSA antibodies. These cases had complete AV block without disruption of the tensor apparatus of the AV valves. The tissue blocks had been serially cut at 10 μm, and sections at 250-μm intervals were stained with Masson’s trichrome. From the remainder, we retrieved representative sections of the AV valves and stained them with elastic-Van Gieson stain for examination of the leaflet architecture. These cases were examined for comparison with patient 1.
We obtained maternal serum from patients 1 and 2 and the previously published patients 3 and 4 for Ro52, Ro60, and La antibody testing. Using the INNO-LIA ANA Update (Innogenetics, Ghent, Belgium) line immunoblot assay according to the manufacturer’s instructions, Ro52 antibodies and antibodies to a specific epitope encoded within amino acid 200-239 (p200) of Ro52 were quantified using enzyme-linked immunosorbent assay with recombinant, bacterially expressed, and purified Ro52 antigen encoded in the pMAL vector system and a synthetized p200 peptide, as previously described. The sera were tested at a dilution of 1:500, and bound antibodies were detected by affinity-purified alkaline phosphatase-conjugated anti-IgG antibodies using p-nitrophenyl phosphate disodium salt as a substrate and registration of the optical density at 405 nm. Sera with high and low Ro52 and p200 antibody levels and mouse anti-human Ro52 monoclonal antibodies generated by us were used as internal controls.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


