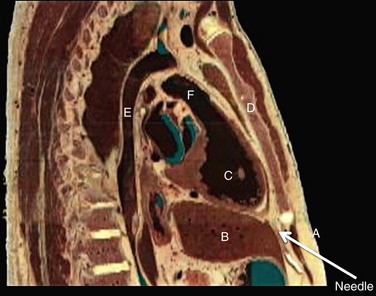
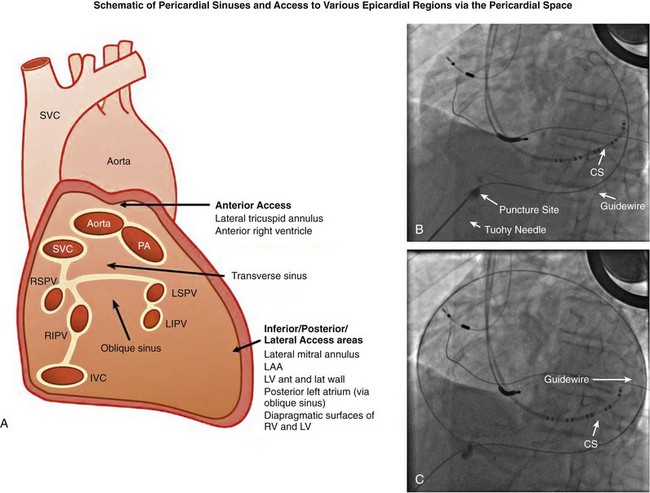
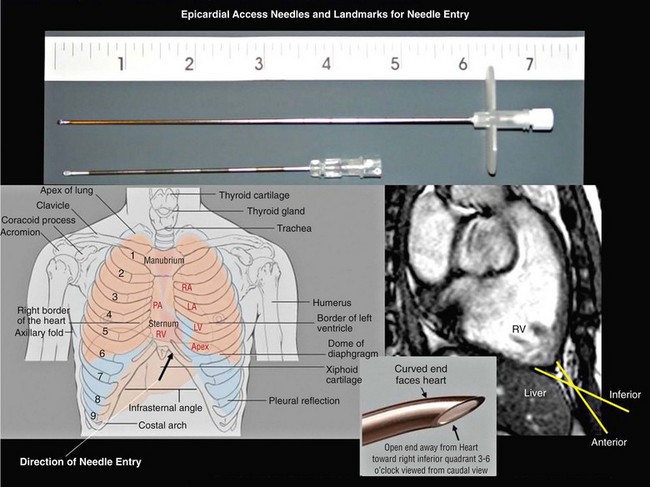
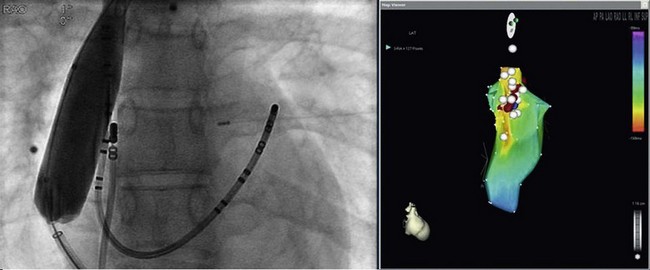
125 The importance of an epicardial approach for the treatment of arrhythmias was not considered until the year 1996, when Sosa et al.1 first described this approach for the treatment of patients with Chagas cardiomyopathy. Before 1996, endocardial mapping and ablation represented the standard approach for the ablation of arrhythmias.2–4 Until that time, open chest surgery was the only alternative to endocardial ablation failures as described in 1969 for the surgical resection of the bypass tract in patients with Wolff-Parkinson-White syndrome.5,6 The importance of epicardial mapping and ablation after the series described by Sosa et al.1 has continued to grow for both the treatment of supraventricular and ventricular arrhythmias. Epicardial access is currently used in all experienced electrophysiology laboratories as an adjunctive strategy for challenging arrhythmias, either after the failure of conventional endocardial ablation or as first line approach.7–32 A survey of ventricular tachycardia (VT) tertiary referral centers showed that epicardial access was necessary in 19% of patients undergoing VT ablation,33 ranging from 6% in healthy hearts to 41% in patients with arrhythmogenic right ventricular dysplasia (ARVD). The oblique sinus is attached anteriorly to the atria and inferiorly to the vena cava. By navigating in the oblique sinus, epicardial mapping and ablation of both atria and ventricles can be achieved.34–41 It is the author’s opinion that epicardial instrumentation should not be attempted in subjects with previous cardiac surgery. Differently, in the case of arrhythmia recurrence following an epicardial ablation, the epicardial space can be safely reaccessed.42,43 Partial and, more rarely, total absence of the pericardium have been described in 1 : 10,000 autopsies and are often associated with other cardiac anomalies. In case of partial defect of the pericardium, a foramen of variable size connects the pericardial and the pleural cavity. In case of the absence of the pericardium, heart and lungs occupy a common serous cavity (75% of cases).44 As described before, the pericardial space contains 15 to 50 mL of serous fluid providing the key to access the pericardial space in patients without pericardial effusions. Sosa et al.1 first reported the subxiphoid percutaneous puncture to access the virtual space between the heart and the pericardium The subxiphoid approach is the best known and most frequently used for percutaneous access although other approaches such as parasternal or apical can also be used.37 In addition, transesophageal,45 transatrial,46,47 and transbronchial48 methods to access the pericardial space have also been described. Two different puncture site orientations have been described: • Anterior—directing the needle superiorly with a shallow trajectory to enter the pericardial space anteriorly over the right ventricle (RV) and to allow easy maneuverability over the left ventricle (LV) • Inferior—directing the needle with a steeper trajectory toward the left shoulder, thus entering the epicardial space over the basal, inferior part of the ventricles (Figures 125-1, 125-2) Figure 125-1 Anatomic sagittal section crossing the middle of the sternum. The needle to achieve pericardial access is inserted in the substernal plane. A, Skin, subcutaneous fat, and rectus abdominis are shown. B, Liver. C, Right ventricle. D, Sternum. E, Aorta. F, Right ventricular outflow tract. (Reproduced from the Visible Human Server.) Figure 125-2 A, Reconstruction of the pericardial sinuses. B and C, Fluoroscopic images of the pericardial access showing the pericardial puncture in left anterior oblique view using an inferior approach. The guidewire loops around the heart on both side of the heart, confirming the successful epicardial access. SVC, Superior vena cava; PA, pulmonary artery; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; LAA, left atrial appendage; RV, right ventricle; LV, left ventricle. (Modified with permission by Drs. Noel G. Boyle and Kalyanam Shivkumar.) The skin in the subxiphoid region is sterilized and anesthetized with lidocaine 1%.8 A 17-gauge standard Tuohy needle (marketed by multiple manufacturers, originally designed for epidural access by Havel’s, Cincinnati, OH, and BD Medical, Franklin Lakes, NJ), either 3.5 or 6 inch in length, is commonly used to perform the puncture (Figure 125-3). Figure 125-3 Representation of the Tuohy needle, 3.5 or 6 inches long, commonly used to perform the puncture. A schematic representation of the landmarks used for needle access is shown, together with the needle orientation, while accessing the epicardium. The yellow arrows in the magnetic resonance imaging scan show the possible different access orientation (anterior or inferior) when performing the access. (Left lower panel image by Patrick J. Lynch, Yale University. Tuohy needle tip image obtained from http://www.ganfyd.org.) The puncture of the fibrous pericardium is appreciated with a release of resistance on the needle (sometime a pop can be felt), and the injection of contrast will result in a contrast layer outlining the heart in the pericardial space. A long guidewire is then advanced into the pericardial space, and in the left anterior oblique (LAO) view it is observed to follow the left cardiac border and preferably cross from the left to right side in front of the great vessels, confirming the pericardial location. Before advancing the sheath, it is extremely important to see the guidewire looping several times around the cardiac silhouette and all the chambers of the heart in the LAO view. The right anterior oblique–anteroposterior projections can be misleading because a perforation of the RV can lead the wire in the pulmonary artery or in the right atrium, and this can simulate an intrapericardial location of the wire.35–41 The advent of deflectable sheaths (Agilis EPI; St. Jude Medical, Minnetonka, MN) and robotic magnetic navigation has improved the ability to navigate in the pericardial space.49 Several major complications can occur during epicardial access, mapping, and ablation. When the pericardial space is accessed, liver, colon, and diaphragm with its vascular supply can be injured. The needle might inadvertently enter and puncture the right ventricle, pleural space, or lung. The use of the curved-tip Tuohy needle is helpful to decrease the risk of entering the RV and direct the guidewire when the epicardial space is entered. In a preliminary series of three patients, no acute complications were reported1 and there was no effusion at the follow-up echocardiogram. In a recent report, there were puncture-related complications such as right ventricular puncture not requiring intervention in 4.5% of the cases, hemopericardium requiring drainage in 7% of the cases, and one occlusion of a marginal coronary artery resulting in a non–Q wave myocardial infarction.50 Avoiding coronary artery damage is another major concern when ablating in the epicardial space. A coronary angiogram can be performed to delineate the location of the coronary arteries. Radiofrequency (RF) energy should be delivered when the distance between the catheter tip and a visible coronary artery is at least 0.5 to 1 cm. This distance is primarily based on operator experience8 and a few published studies.51 The authors of this chapter do not routinely perform angiograms in patients with structural heart disease and have not experienced complications.52 Phrenic nerve injury is another possible complication that can be minimized using high output pacing at the ablation sites. Sosa et al. reported no cases of phrenic nerve injury by using high output pacing (15 mA, 5 ms pulse).7,51 Pericarditis without pericardial effusion is another possible complication reported by Sosa in 30% of the patients after the procedure; it responded to treatment with antiinflammatory drugs.7,43,51,53 Sacher et al.33 reported a multicenter safety study from three tertiary centers performing epicardial VT ablation in 2010.33 There were eight (5%) acute major complications related to epicardial access: seven epicardial bleeding and one coronary stenosis. Three delayed complications occurred after 48 hours: one major pericardial inflammation, one tamponade (10 days after) and one acute inferior myocardial infarction (2 weeks after). No constrictive pericarditis or phrenic nerve injuries were reported. In a recent European multicenter study,53 major complications were observed in nine (4.1%) patients, (cardiac tamponade in eight and abdominal hemorrhage in one). Minor complications such as heart failure, intermittent atrioventricular block, and pneumonia were observed in 17 patients (7.8%), whereas 10% of the patients experienced severe pericardial pain after the procedure. It is common to prescribe nonsteroidal antiinflammatory medications.54 Unusual complications have been reported in a recent series of 334 patients with subcapsular hepatic hematoma, coronary spasm, and RV pseudoaneurysm that were all managed conservatively. Surgical intervention was required for cases of liver puncture with intraabdominal bleeding, pericardial bleeding owing to middle cardiac vein laceration, pericardial bleeding owing to coronary sinus laceration, and RV abdominal fistula. Other uncommon complications such as a pleuropericardial fistula can occur and should be considered if a new left pleural effusion occurs after the procedure.55,56 Recently, it has been shown in a series of 30 patients that repeated pericardial access can be performed safely at a median of 110 days after the initial procedure.42 However, a case of constrictive pericarditis requiring pericardiotomy after a fourth epicardial ablation procedure, resulting in severe heart failure, has been reported as well.43 Other methods of improving fluoroscopy orientation include the positioning of mapping catheters (e.g., aortic root, right ventricle, coronary sinus), and the use of intracardiac echocardiography.34 Different techniques have been described to avoid phrenic injury. Buch et al.57 used a Meditech balloon (Boston Scientific, Natick, MA) to displace the phrenic nerve on the lateral epicardial wall protecting it during RF ablation. Fan et al.58 used a peripheral angioplasty balloon to separate the phrenic nerve from the ablation target site in a patient with nonischemic cardiomyopathy.22 Our group compared different methods to separate the phrenic nerve from the epicardial surface during both epicardial and endocardial RF ablation. In many cases, the use of air and saline resulted in the best strategy to avoid phrenic nerve capture. The use of a peripheral balloon was a valid alternative.59 In this regard, the successful deployment of a balloon in the epicardial space has improved with the use of deflectable sheaths. Epicardial balloons were used for esophageal protection during ablation of the posterior wall and to displace the phrenic nerve during endocardial ablation in a case of inappropriate sinus tachycardia.59–61 Protection of the coronary arteries is key when ablating in the epicardial space.62 There are few data available in addition to the seminal experience of Sosa et al.1 D’Avila et al.63 studied the effects of RF lesions delivered in the vicinity of coronary arteries; they found that the risk of vascular damage varied inversely with vessel size. This finding implies that larger vessels might be protected by increased blood flow and possibly epicardial fat.63 Thyer at al.64 showed that the instillation of intracoronary chilled saline (5°C) during RF ablation over the coronary artery in excised sheep hearts decreased the temperature in the vessel and the distance between the lesion and the artery. Viles-Gonzalez et al.65 delivered RF lesions within 1 mm of the coronary arteries in seven pigs, and they examined the acute (20 days) and chronic (70 days) effects.65 In both groups, intimal and medial thickening was seen, with replacement of smooth muscle cells by extracellular matrix. Whether these findings can be applied in humans requires further investigation. The safest approach is to avoid RF delivery within 1 cm of an epicardial coronary artery. However, in patients with structural heart disease, it may be safe to perform epicardial ablation within the scar area without the need for coronary angiography.52,65 New tools and technologies to reduce complications and increase feasibility of the epicardial access will be discussed in a following paragraph. The first reported ablation of an accessory pathway used the open surgical approach in 1969.6 With the development of endocardial RF catheter ablation, the majority of bypass tracts have been treated with percutaneous RF ablation. The coronary sinus66,67 or a coronary sinus diverticulum68,69 can provide a route to locate and ablate epicardial pathways, but there are some pathways that cannot be reached via the coronary sinus or its branches. Several uncommon locations such as posteroseptal and left posterior pathways as well as right atrial appendage to right ventricular pathways and left atrial appendage to left ventricular pathways have been described as challenging pathways requiring epicardial ablation to achieve success.6,10–16 Combined epicardial and endocardial approaches have been used when endocardial or epicardial approaches alone were unsuccessful.15 Morady et al.10 reported that in 8% of the cases the reason for endocardial ablation failure of an accessory pathway was the epicardial location of the AP. In these cases, epicardial mapping and ablation should be considered. The epicardial approach can include ablation within the coronary sinus and its tributaries. Importantly, it has to be mentioned that in some cases epicardial ablation might fail because of the presence of a thick fat pad, because pathways are annular in location, or for the inability to deliver energy in close proximity to collateral structures such as the coronary vessel or the phrenic nerve. Schweikert et al.14 and Valderrábano et al.15 reported a series of patients with accessory pathways and previously failed endocardial ablations who had successful epicardial ablation. A few cases or series have reported on epicardial ablation of atrial tachycardia usually due to uncommon locations such as the right and the left atrial appendages.17,23 Epicardial access could be required to inflate an angioplasty balloon in the pericardial space to avoid phrenic nerve injury during endocardial ablation at the level of the crista terminalis. Ablation of inappropriate sinus tachycardia is challenging because the sinus node has a subepicardial location and frequently is in close proximity to the phrenic nerve. Endoepicardial ablation has been used successfully by different groups to achieve sinus node modification and to avoid phrenic nerve palsy. In this regard, an angioplasty balloon or the use of saline and air have been used as possible ways to increase the procedural success while minimizing the risk for phrenic nerve damage (Figure 125-4).18–22 Figure 125-4 Case of inappropriate sinus tachycardia (IST) in a 19-year-old woman referred to the authors’ institution after a failed ablation. Because the ablation target electrograms were at the level of the phrenic nerve (PN; verified by high output pacing), a peripheral balloon was placed epicardially and inflated at the level of the sinus node to separate the PN from the myocardium. The IST was successfully ablated endocardially with radiofrequency energy. The left panel shows an anteroposterior fluoroscopy view of the ablation catheter and the peripheral balloon at the level of the sinus node. The right panel shows a three-dimensional reconstruction of the right atrium in left lateral (LL) view. The white dots indicate the phrenic nerve location, and the red dots indicate the ablation points overlapping the phrenic nerve area. Few data are available regarding ablation of atrial fibrillation using an epicardial approach. The sinuses and reflections in the pericardial space prevent complete encircling with isolation of the pulmonary veins.70 The vein of Marshall is a potential nonpulmonary vein trigger of atrial fibrillation. This structure can be accessed epicardially or through the coronary sinus.24 Hwang et al.71 reported six cases in which the AF was triggered by the vein of Marshall and epicardial ablation via the coronary sinus was able to achieve stable sinus rhythm.71 Similarly, Katritsis et al.72 showed patients were the sole focus of AF was epicardial and it was ablated via the coronary sinus. Pak et al.25 showed the feasibility of adjunctive epicardial ablation to achieve success in persistent AF cases. All patients had failed previous endocardial ablation and underwent adjunctive ablation sites such as roof, perimitral annulus, and ligament of Marshall. Such areas can require long RF times and are more amenable to be treated with epicardial ablation. Kowalski et al.73 performed epicardial mapping in a series of patients undergoing surgical Maze procedure within 8 ± 11 months after the endocardial ablation. Interestingly, they showed PV conduction in the epicardial region in 62.5% of the cases.73 In a recent case of persistent atrial fibrillation, a combined endocardial-epicardial approach was used to create a box set of lesions on the posterior atrial wall isolating all four PVs and the posterior wall together.74 Scanavacca et al.75 performed endocardial or epicardial RF ablation, or both, in seven patients in which high-frequency stimulation (20 Hz) induced vagal reflexes (atrioventricular block > 2 seconds) without pulmonary veins isolation. The authors reported a high recurrence rate.75 Given the small number of patients, additional studies are needed to determine whether this approach has a role in AF ablation procedures. Kiser et al.26 reported on 28 patients with persistent or LSP AF undergoing a combined surgical epicardial radiofrequency ablation and endocardial ablation. Despite the enthusiasm for the short-term results, the long-term follow-up showed no improved success compared with manual ablation with a higher complication rate. Although the idea of a hybrid approach remain interesting, the technology used in the series by Kiser et al.26 is not advisable because of a dismal success rate at follow up. Mahapatra et al.27 reported on the initial experience of 15 patients with persistent and long-standing persistent atrial fibrillation undergoing surgical epicardial and endocardial ablation with the “atri cure” technology following a failed endocardial ablation. At 20.7 ± 4.5 months of follow-up, 87% of the patients were free from atrial tachyarrhythmias. When planning a VT ablation, an epicardial origin should always be considered. Several groups sought to determine electrocardiogram (ECG) criteria to predict an epicardial origin of the VT.28 However, often the 12-lead ECG can be misleading because of the presence of delayed activation around scar regions that might confound the ECG morphology. In addition, the majority of patients with ischemic cardiomyopathy and VTs are treated with amiodarone or other antiarrhythmic drug (AAD) treatment, which might jeopardize the sensitivity and specificity of those criteria. Berruezo et al.76 analyzed the ECG recordings of patients with VT successfully ablated from the epicardial LV. They showed that the presence on the ECG of a pseudo delta wave of 34 ms or greater (measured from the onset of ventricular activation to the onset of the earliest rapid deflection in any precordial lead), an intrinsicoid deflection time of 85 ms or greater (measured from the onset of ventricular activation to the peak of the R wave in lead V2), and an RS complex duration of 121 ms or greater were predictors of an epicardial origin of the VT with an overall sensitivity greater than 80% and a specificity of approximately 85%. It is important to note that 64% of the patients had ischemic CM and that a more recent analysis showed that none of these criteria could reliably predict an epicardial VT focus.77 The Marchlinski group78,79 sought to determine the epicardial origin of VTs in patients with nonischemic cardiomyopathy. They showed that the majority of LV VTs with an endocardial origin present small Q waves in the inferior leads and small R wave in lead I, expressing the depolarization of a small segment of myocardium with an endo- to epi-activation. In contrast, when the VT has an epicardial origin, it will not show an initial Q wave in the inferior leads and will consistently show a Q wave in lead I. In the RV, when the stimulus rises from the epicardial surface, the QRS will show an initial negative force (Q waves) in inferior leads, lead I, or lead V2, depending on the origin. The anterior epicardial sites will show Q wave or QS complex in lead I and V2, and the inferior RV epicardial sites show an initial Q wave in the inferior leads.79 Of note, left-sided outflow tract ventricular tachycardia (LVOT-VT) with an epicardial origin can be ablated from the coronary cusps. In this setting, the R wave duration index and R/S amplitude index in lead V1 or V2 are useful ECG criteria to identify Epi-LVOT-VT. In this scenario, a delayed precordial maximum deflection index of 0.55 or greater identifies epicardial LVOT-VT far from the aortic sinus of Valsalva, with a sensitivity of 100% and a specificity of 98.7% relative to all other sites of origin. The Q wave ratio of lead aVL to aVR and S wave amplitude in lead V1 are useful information to distinguish an Epi-LVOT-VT originating from the LV epicardium far from the left sinus of Valsalva and VTs from the left sinus of Valsalva. However, it can be concluded that ECG criteria to identify epicardial VT are substrate specific and that in many cases can be misleading. It appears that the specificity and sensitivity is higher in idiopathic nonischemic substrate (Figure 125-5). Epicardial mapping in the pericardial space is similar to the one performed on the endocardial surface. Areas of scar with abnormal electrograms (low amplitude, delayed or fractionated potentials) should not be confused with epicardial fat that can simulate area of scar.80,81 For those reasons, a voltage less than 1 mV is used to define scar in the pericardial space differently from the endocardium where scar is anything less than 0.5 mV. Epicardial fat can also represent an obstacle for pacing (entrainment and pace mapping) as well as for ablation. For these reasons, high pacing outputs are often required. In the pericardial space, radiofrequency energy without irrigation will be limited by a lack of cooling because of the absence of circulating blood, resulting in temperatures at low power settings. D’Avila et al.82 compared the efficacy of standard versus cooled-tip RF catheters during epicardial ablation in animals showing that standard RF lesions using a 4-mm tip catheter was less effective in producing deep lesion than the RF-irrigated tip catheter. In addition, in fat area standard RF did not generate any appreciable lesion, and 4-mm lesions were created with the irrigated catheter.82
Special Ablation Approaches
Epicardial, Other
Anatomy of the Pericardial Space
Pericardial Anomalies
Methods of Accessing the Pericardial Space
Subxiphoid Approach
Complications
How to Avoid Complications
Epicardial Ablation of Supraventricular Tachycardias
Accessory Pathways
Atrial Tachycardia
Inappropriate Sinus Tachycardia
Atrial Fibrillation
Epicardial Ablation of Ventricular Tachycardias
When to Consider an Epicardial Approach for Ventricular Tachycardia Ablation
Mapping, Ablation, and Energy Sources in the Pericardial Space
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Special Ablation Approaches: Epicardial, Other