Small Vessel and Diffuse Disease
Mladen I. Vidovich MD, FACC, FSCAI
Percutaneous coronary intervention (PCI) in small vessels (SVs) remains one of the most challenging aspects of current interventional practice. Along with diabetes mellitus and diffuse coronary artery disease PCI has traditionally been associated with increased restenosis rate, reduced success rates, overall increased complication rates, higher target lesion revascularization (TLR) and consequently increased major adverse cardiac event (MACE) rates (1, 2, 3 and 4). Similar to percutaneous revascularization, in-hospital mortality after coronary artery bypass grafting (CABG) is higher in patients with small coronary arteries (5).
The issue of SV PCI has undergone substantial change since the advent of angioplasty and can be generally divided in three phases: balloon angioplasty (PTCA), bare-metal stenting (BMS) and contemporary drug-eluting stenting (DES). In addition, several novel approaches to SV disease will be discussed—drug-eluting balloons (DEB) and local drug delivery, systemic antiproliferative agents and emerging use of bioabsorbable stents.
DEFINITION
Currently, there is no accepted definition for “SVs.” Nonetheless, vessel sizes <2.75 mm or <3.0 mm have traditionally been accepted by most interventionalists and researchers in this field as “SV” size. Historically, SV PCI was performed in approximately 30% to 50% of interventions; (2, 6) however, contemporary “all-comers” clinical trials have enrolled a substantially higher proportion of patients with SV disease. These trials include broader patient populations with a high percentage of “off-label use” and may be more representative of patients and lesions encountered in everyday clinical practice. Both in the Resolute All-comers trial which compared the everolimus eluting stent (EES) with the zotarolimus eluting stent (ZES) platforms, and in the LEADERS trial (biolimus platform) almost 68% of patients had reference vessel diameter ≤2.75 mm (7, 8). Thus, such a large proportion of SV PCI represents an important clinical and economic consideration. The availability of modern, thin-strut DES in 2.25-mm diameter sizes has facilitated the continued search for solutions for PCI in ever smaller vessels. This is well illustrated with the contemporary XIENCE Nano trial that enrolled patients with lesions in vessels between 2.25 and 2.5 mm in size (9). It is clear that, as the PCI and stent technology advance, our definition of what “small vessel” represents will continue to evolve.
BALLOON ANGIOPLASTY VERSUS BMS
After the initial success of PTCA in large vessels it was quickly recognized that PTCA for SVs disease was associated with considerable difficulties, particularly frequent restenosis requiring repeat interventions. SV size was earlier identified as an independent risk factor contributing to vessel restenosis (2). Since the amount of neointimal hyperplasia is largely independent of vessel size (10), the late loss is similar in small and large vessels (11). Conversely to late loss which is the absolute measure of restenosis, relative measures of restenosis, such as percent diameter stenosis, are directly dependent on vessel size. Therefore, since the acute gain achieved in SVs is much smaller than in large vessels, the net lumen gain in turn decreases to a larger degree and therefore carries a higher rate of restenosis (12) (Fig. 24-1).
After studies performed in large vessels (>3.0 mm) demonstrated that BMS was superior to PTCA regarding restenosis, interest and research among interventionalists had gradually shifted to SVs.
In an initial study, BMS PCI in large vessels (>3.0 mm) was compared with SVs (<3.0 mm) and demonstrated higher restenosis in the SV group (32.6% vs. 19.9%, p < 0.0001). A conceptually very important finding was that the late loss was similar in the two groups (SVs, 1.11 ± 0.85 mm vs. large vessels, 1.05 ± 0.91 mm, p = NS). Predictors of freedom from restenosis were larger postprocedure minimal stent cross-sectional area (OR 1.190, p = 0.0001) and shorter lesions (OR 1.037, p = 0.01). At one-year follow-up, patients with SVs also had a lower rate of event-free survival (63% vs. 71.3%, p = 0.007) (3).
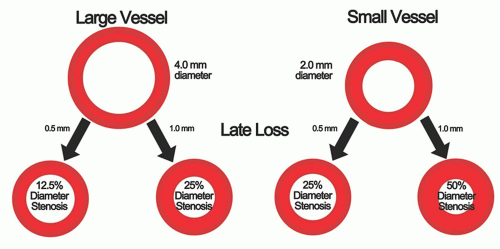 FIGURE 24-1 Impact of late loss on diameter stenosis in large and small vessels. Equivalent late loss in different size vessels causes significantly different percent diameter stenosis. |
TABLE 24-1 Representative Trials of BMS versus PTCA in Small Vessel Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subsequently, numerous trials were performed to determine the role of stent placement in SVs when compared with balloon angioplasty alone. Major findings of some of the representative trials are summarized in Table 24-1 (13, 14, 15, 16 and 17).
The accumulated knowledge gained from those studies is well summarized in two major meta-analyses performed at that time. The meta-analysis by Agostoni et al. included 4,383 patients from 13 trials with reference vessel size <3.0 mm. One of the most important findings was that neither death (OR 0.81, 95% CI, 0.48-1.36) nor myocardial infarction (MI) (OR 0.80, 95% CI: 0.58-1.11) was different between the BMS and PTCA groups. However, MACE (OR 0.71, 95% CI: 0.57-0.90) were favoring the BMS stenting approach (17.6%) compared with the PTCA strategy (22.7%). Decreased MACE were driven mainly by reduced repeat revascularization (OR 0.76, 95% CI: 0.61-0.95). Notably, in further analyses, optimal PTCA had similar MACE to BMS stenting (OR 0.84, 95% CI: 0.63-1.12) while suboptimal PTCA was associated with significantly worse MACE than BMS (OR 0.53, 95% CI: 0.37-0.76, p < 0.001) (18).
The meta analysis by Moreno et al. included 3,541 patients from 11 studies. SVs were similarly defined as <3 mm in diameter. The restenosis rate across those studies was 25.8% for BMS stenting and 34.2% in patients allocated to the PTCA strategy (RR 0.77, 95% CI: 0.65-0.92, p = 0.003). Patients receiving BMS had lower MACE rates (15.0% vs. 21.8%, RR 0.70, 95% CI: 0.57-0.87, p = 0.002) and less target vessel revascularizations (TVR) (12.5% vs. 17.0%, RR 0.75, 95% CI: 0.61-0.91, p = 0.004). There were no differences in death or MI. The overall conclusion was that elective BMS stenting was superior to provisional stenting (19).
Strut Thickness and BMS
The ISAR-STEREO-2 trial was an initial study performed in large vessels comparing two different BMS platforms-a thin-strut stent (50 µm) with a thick-strut stent (140 µm) and a significant decrease was found in angiographic restenosis in the thin-strut group (17.9%) when compared with the thick-strut group (31.4%) (RR 0.57, 95% CI: 0.39-0.84, p < 0.001) with no difference in death and MI at 1 year (20). This was further confirmed in an analysis that encompassed several stent platforms. In this analysis thin-strut stents were defined having strut thickness <100 µm. Restenosis was significantly lower in the thin-strut group (28.5%) compared with the thick-strut group (36.6%) (21). Somewhat discordant with these and subsequent studies investigating the effect of strut thickness on outcomes, a large meta-analysis did not demonstrate any association between strut thickness and restenosis reduction, likely due to smaller differences in strut thickness among the trials (19).
Importantly, when a thick-strut drug-eluting stent (Cypher 140 µm, sirolimus-eluting stent) was compared with a thin-strut bare metal stent (BeStent 76 µm) angiographic restenosis rate was 8.3% for the Cypher stent and 25.5% for the BeStent (p < 0.001) further underlining the importance of anti-proliferative therapies in SVs and the high risk of these lesions even when treated with a thin-strut BMS (22).
Heparin Stent Coating with BMS in Small Vessels
Before the introduction of contemporary anti-proliferative stent technologies, attempts were made to study the effect of heparin coating in the prevention of restenosis and stent thrombosis. COAST was a randomized trial in which PTCA was compared with uncoated BMS and with BMS with heparin coating in SVs ranging from 2.0 to 2.6 mm. Restenosis rates were found to be similar (32%, 25% and 30%, respectively) with similar stent thrombosis rates (1.0% for PTCA and 0.5% for BMS or heparincoated BMS) (23).
Cilostazol and BMS in Small Vessels
Given overall high rates of restenosis associated with BMS in SVs additional efforts in providing systemic pharmacological therapy have yielded somewhat positive results. Subgroup analysis of 316 patients with vessel diameter <2.75 mm in the CREST trial demonstrated that restenosis in the group that received cilostazol 100 mg twice daily in addition to dual antiplatelet therapy with aspirin and clopidogrel had lower restenosis rates compared with the traditional arm (23.6% vs. 35.2%, RR 0.67, 95% CI: 0.47-0.95). Nonetheless, these rates remained significantly higher than those that could be achieved with drug-eluting stents (24).
IVUS and BMS in Small Vessels
Since the acute gain is less in SVs, use of intravascular ultrasound (IVUS) and IVUS-guided stent post dilation would be expected
to improve outcomes in SV PCI. This hypothesis was tested in a study that retrospectively analyzed patients who underwent SV PCI (reference diameter <2.75 mm) with IVUS-guidance. Based on final IVUS lumen areas, two groups were identified and prospectively followed, ≤6.0 mm2 and >6.0 mm2 lumen area groups. TLR was significantly lower in the larger lumen group (39% vs. 26%, p = 0.01) and MACE non-significantly reduced (44% vs. 34%, p = 0.07). Death and MI were similar. A final IVUS cross-sectional area of ≤6.0 mm2 was independently associated with higher TLR (OR 1.84, 95% CI: 1.23-2.51, p = 0.01) (25).
to improve outcomes in SV PCI. This hypothesis was tested in a study that retrospectively analyzed patients who underwent SV PCI (reference diameter <2.75 mm) with IVUS-guidance. Based on final IVUS lumen areas, two groups were identified and prospectively followed, ≤6.0 mm2 and >6.0 mm2 lumen area groups. TLR was significantly lower in the larger lumen group (39% vs. 26%, p = 0.01) and MACE non-significantly reduced (44% vs. 34%, p = 0.07). Death and MI were similar. A final IVUS cross-sectional area of ≤6.0 mm2 was independently associated with higher TLR (OR 1.84, 95% CI: 1.23-2.51, p = 0.01) (25).
Stent Thrombosis and SV PCI
A large study that included a very broad population of unselected and consecutive patients offered early understanding of factors associated with stent thrombosis in SV PCI (4). In this study, average vessel size was 2.6 mm and 30-day stent thrombosis was 4.2%. Residual dissection (OR 5.38), reduced left ventricular (LV) function (OR 3.08), and acute coronary syndrome (OR 2.53) were some of the factors associated with thrombotic events. Nowadays, the findings of this study are somewhat of historical interest as contemporary dual antiplatelet therapy and devices were not used at that time contributing to the high observed rates of stent thrombosis.
Balloon Angioplasty and Adjunctive Devices
Before the introduction of DES, research had focused on alternative strategies to reduce high rates of restenosis in SVs. Interestingly, as early as 1993, in a landmark study, it was recognized that late outcome after PTCA, BMS and directional atherectomy depended mainly on the immediate results and not on the procedure per se used to obtain the result (26). Although not specifically describing SV use, this important concept was confirmed in a large meta-analysis in 2004 (27). To test whether these adjunctive devices were a possible solution to high MACE rates in SVs and to test the hypothesis whether reduction of plaque burden was beneficial in SVs, several trials that focused on those devices were performed.
Rotational Atherectomy in SV Disease
The value of rotational atherectomy compared with PTCA in SVs (mean reference diameter 2.46 mm) was reported in the DART trial. Target vessel failure (composite of death, Q-wave MI and clinically driven revascularization) was similar in the rotational atherectomy and PTCA groups (30.5% vs. 31.2%). Both acute gain (rotablation 0.86 mm vs. PTCA 0.88 mm) and late loss were similar (rotablation 0.49 mm vs. PTCA 0.56 mm) (28).
Cutting Balloon in SV Disease
Similarly, the use of cutting balloon in SVs was investigated in a large randomized trial (mean reference diameter 2.86 mm). This study demonstrated essentially equivalent six-month binary angiographic restenosis in the cutting balloon group (31.4%) compared with the PTCA group (30.4%). There were statistically more perforations (0.8% vs. 0%) and higher rates of MI, death and total MACE in the cutting balloon group (4.7% vs. 2.4%), however (29). Despite the overall equivalence between PTCA and these adjunctive techniques, these trials demonstrated that their use remains safe and equally effective in lesions subsets that are more amenable to rotational atherectomy (e.g., heavily calcified lesions) or cutting balloon use (e.g., fibrotic lesions prone to recoil or “watermelon seeding effect”).
In summary, the two large meta-analyses and subsequent specialized trials had solidified our understanding on the impact of BMS PCI and outcomes of PTCA in SVs. Compared with today’s DES, restenosis rates were high, albeit lower, with BMS stenting. It is important to emphasize that even in today’s practice where modern medical therapy has been shown to be extremely important in the treatment of coronary artery disease (30) optimal PTCA may in certain clinical scenarios offer quite satisfactory results compared with BMS. SV coronary stenting with BMS reduces the restenosis rate with subsequent fewer MACE. The reduced MACE is driven mainly by reduced target vessel revascularization. There is no difference in MI and death. Bare metal stents with thinner stent struts are associated with lower restenosis rates.
First-Generation DES
Sirolimus-Eluting Stent
SIRIUS 2.25 trial was a small nonrandomized study that used propensity-matched PTCA and BMS historical controls. Yet, it easily demonstrated a remarkable reduction in 6-month TLR with SES (4.0%) versus the Bx Velocity BMS (15.0%). In-lesion binary restenosis was similarly reduced to 16.9% in the SES group compared with the 30.6% to 45.9% range in the historical BMS controls (31).
Subsequently, in a randomized prospective fashion, the SESSMART trial compared SES versus BMS in vessels with mean vessel diameter of 2.2 mm. The binary in-segment restenosis after 8 months was 53.1% in the BMS group and 9.8% in the SES group (RR, 0.18; 95% CI: 0.10-0.32; p < 0.001). Similarly, MACE was significantly reduced in the SES group 9.3% versus 31.3% in the BMS group (RR, 0.30; 95% CI: 0.15-0.55; p < 0.001) mainly because of a reduction in TLR (7% vs. 21.1%) and MI (1.6% vs. 7.8%) (32).
Paclitaxel-Eluting Stents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


