Sleeve Lobectomy
L. Penfield Faber
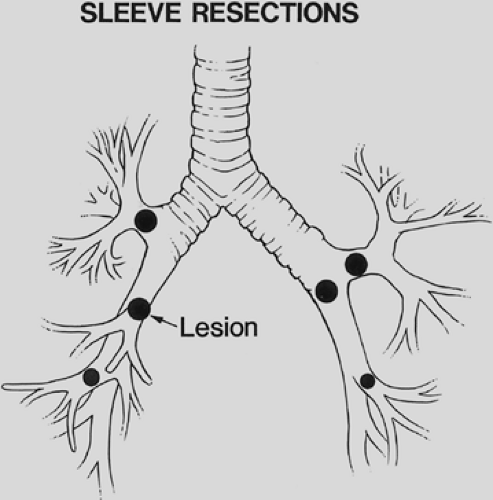
A sleeve lobectomy is the removal of a portion of a main bronchus in conjunction with the involved lobar bronchus and associated lung tissue (Fig. 29-1). Sleeve lobectomies can be accomplished in selected patients with specific technical criteria:
The bronchial margin must be free of microscopic cancer.
A complete resection of all bronchial and lymphatic cancer must be achieved.
The main pulmonary artery must be preserved.
The tumor must be confined to the resected portion of lung.
A sleeve lobectomy is the alternative to a pneumonectomy for lung cancer; when compared with pneumonectomy, it is achieved with less morbidity and mortality and similar long-term results.
In 1947, Price-Thomas28 carried out the first sleeve lobectomy for a bronchial adenoma, which was reported by him in 1956. A bronchoplastic wedge resection of a portion of the left main bronchus for a bronchial adenoma was then accomplished by D’Abreu and McHale3 in 1949 and reported by these surgeons in 1951. Allison1 in 1959 reported the first successful sleeve lobectomy for carcinoma and was the first to report resection and reconstruction of a portion of the adjacent pulmonary artery infiltrated by tumor in the patient undergoing sleeve lobectomy for carcinoma. Price-Thomas29 summarized the role of sleeve resection in selected patients with lung cancer in 1960. Paulson and Shaw27 stressed the importance of preserving functional lung tissue in 18 sleeve resections for benign and malignant lung tumors.
Indications
Sleeve resections are applicable in patients with lung cancer, bronchial carcinoids, other bronchial tumors, benign bronchial strictures related to trauma or inflammatory disease, acute traumatic disruption of the airway, and metastatic malignancies with lobar extension to the main bronchus. Approximately 6% to 8% of resections for primary lung cancer are sleeve resections.
Tumor in a lobar orifice or a lobar tumor invading the main bronchus precludes a standard lobectomy, and sleeve lobectomy becomes the procedure of choice (Fig. 29-2). Preoperative bronchoscopy can identify the need for a sleeve lobectomy by the visualization of tumor in the lobar orifice. If cancer is found at the lobar resection margin on frozen section analysis or there is extraluminal extension of the tumor to the mainstem bronchus, a more extensive resection than standard lobectomy is required. Lymph nodes involved by cancer can invade the bronchial wall at the lobar bronchial margin, and resection of a portion of the main bronchus is required. Resections for second primary lung cancers require conservation of lung tissue, and a sleeve lobectomy can avoid pneumonectomy.
Additional indications for sleeve lobectomy are patients >70 years of age and those with compromised cardiopulmonary function. Sleeve lobectomy avoids a pneumonectomy, the mortality rate is lower with a sleeve procedure, and postoperative quality of life is enhanced. A postoperative predicted forced expiratory volume in 1 second (FEV1) of less than 50% indicates a likelihood of complications following pneumonectomy, and preservation of lung tissue is of benefit. Patients with cardiac disease or other comorbidities always benefit from preservation of lung tissue.
Typical carcinoid tumors are ideally suited for sleeve resections. They frequently have a limited base of invasion into the bronchus, and margins of resections can be relatively close. Long-term results free of disease approach 100%. Patients with mucoepidermoid and adenoid cystic carcinomas are candidates for sleeve resections; a complete resection with accompanying lymph nodes must always be achieved for these lesions.
Kato16 reported on various types of sleeve resection for tuberculous bronchial stenosis. Blunt chest trauma can cause bronchial disruption; if it is not diagnosed acutely, the patient can present months or years later with a benign stricture. Such strictures are located adjacent to a lobar bronchus or in a mainstem bronchus and are ideal for sleeve resection. Major bronchial disruption as a result of penetrating or blunt chest trauma requires debridement of the torn bronchus and reapproximation of viable tissue. Sleeve lobectomy may be required to reconstruct the airway in association with traumatized lung parenchyma.
Benign tumors that require conservation of lung tissue include hamartomas, large lipomas, schwannomas, and granular cell myoblastomas. Metastatic carcinomas can extend to a mainstem bronchus, and sleeve lobectomy is then indicated. This pathologic situation can be particularly applicable to metastatic breast cancer, hypernephromas, and metastatic sarcoma. The one major contraindication to a sleeve lobectomy is when a complete resection for cancer cannot be achieved with the sleeve procedure and the patient cannot tolerate a pneumonectomy. The positive involvement of N2 nodes in a patient with primary bronchial carcinoma as a contraindication to sleeve lobectomy remains controversial.
Surgical Technique
The standard double-lumen tube is the endotracheal tube of choice for the required one-lung anesthesia. Endobronchial balloon blockers can be used for one-lung anesthesia, but bronchoscopy is required for proper positioning and the balloon blockers can be difficult to maneuver. Accurate positioning and the tendency to migrate present intraoperative problems. Hypoxia can occur because of the shunting of unoxygenated blood through the non-ventilated lung. The use of increased FIO2and increased minute ventilation minimizes hypoxia, and occlusion of the main pulmonary artery on the operated side to minimize the shunt is not necessary. Ancillary techniques to improve arterial oxygenation include jet ventilation or continuous positive airway pressure (CPAP) of the operated lung combined with intermittent positive-pressure ventilation of the dependent lung. The anesthesiologist must be familiar with all of these techniques.
The posterolateral thoracotomy is a favorite incision for sleeve lobectomy, but the lateral or vertical axillary thoracotomy is the preferred incision of some. Entrance into the chest through the fifth intercostal space is satisfactory for bronchial sleeve procedures. A pedicled intercostal muscle flap can be mobilized as the chest is opened and is preserved for later bronchial coverage. Sleeve lobectomy has been accomplished by video-assisted thoracic surgery (VATS), but this should be reserved for patients with minimal tumor burden for ease of dissection. This technique should be utilized only by the most experienced VATS surgeon.
The initial dissection deals with the lobar branches of the pulmonary artery to ensure that the resection can be accomplished. If the tumor is in close proximity to lobar branches, proximal control of the main pulmonary artery is recommended. Neoadjuvant therapy can create significant fibrosis with obliteration
of the normal tissue planes of dissection. The tumor may invade the wall of the pulmonary artery or involve the origin of a large lobar branch (Fig. 29-3). In this situation, it may be necessary to carry out a concomitant sleeve resection or patch angioplasty of the pulmonary artery, as described by Schirren33 and Rendina.32
of the normal tissue planes of dissection. The tumor may invade the wall of the pulmonary artery or involve the origin of a large lobar branch (Fig. 29-3). In this situation, it may be necessary to carry out a concomitant sleeve resection or patch angioplasty of the pulmonary artery, as described by Schirren33 and Rendina.32
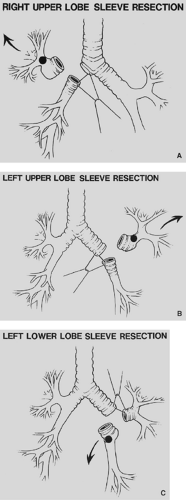 Figure 29-2. A: Right-upper-lobe sleeve lobectomy. B: Left-upper-lobe sleeve lobectomy. C: Left-lower-lobe sleeve lobectomy. |
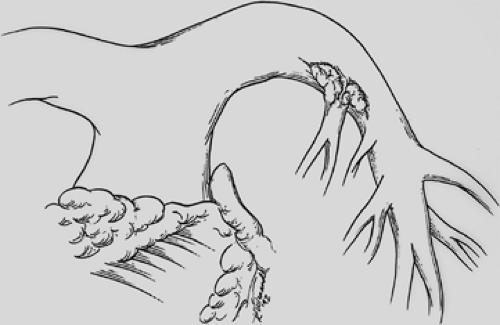 Figure 29-3. Cancer invading the left main pulmonary artery at the origin of the first branch to the left upper lobe. |
Careful dissection is necessary to preserve the bronchial blood supply. Funami12 has clearly described the anatomic course of the bronchial arteries in both the left and right hilum. Preservation of bronchial arteries is also important when carrying out the associated lymphadenectomy. It is preferable to accomplish the lymph node dissection prior to the bronchial sleeve procedure to avoid traction on or manipulation of the anastomosis. Lymph nodes at stations 5, 6, and 7 are in close association with bronchial arteries that are preserved whenever possible. After it has been determined that a complete resection can be accomplished by sleeve lobectomy, the hilar vessels are transected in standard fashion and attention is then directed towards the bronchus.
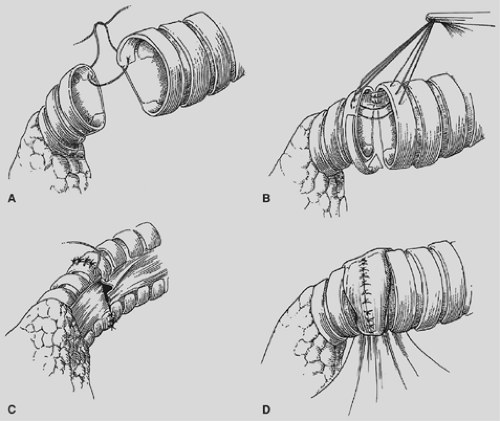
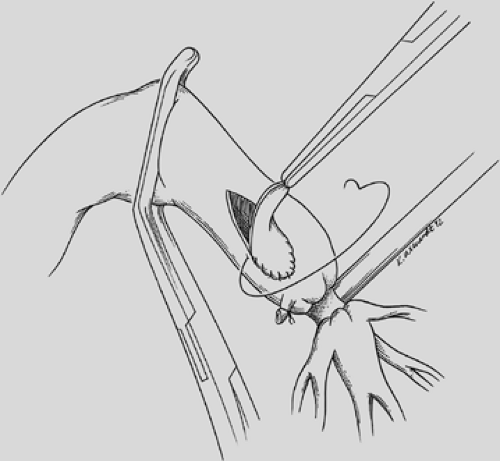
The proximal and distal areas of the main bronchi are circumferentially dissected free of adjacent tissue. A tumor-free margin is selected, and the bronchus is transected with a knife to ensure clean and straight margins (Fig. 29-4).
No attempt is made to cut in any specific area of the membrane or cartilage ring. A straight transection of the main bronchus and associated distal bronchus provides the best opportunity for a complication-free anastomosis. After the specimen is removed (Fig. 29-5), frozen section analysis of both the proximal and distal ends of the bronchus is done to determine the adequacy of cancer resection. A margin positive for microscopic cancer indicates that more bronchus must be resected. Further bronchial resection can create technical problems of anastomotic tension or compromise of a distal bronchus. Careful judgment is necessary, because it may be more appropriate to carry out a pneumonectomy than risk a failed anastomosis with resultant postoperative complications that may be fatal. Techniques to minimize tension on the anastomosis include transection of the inferior pulmonary ligament and also a semicircular transection of the pericardium as described by Vogt-Moykopf.41
We prefer to complete an end-to-end anastamosis with interrupted 4-0 polyglycotic suture material. Two or three through-and-through sutures are initially placed inferiorly to approximate the dependent and medial cartilages. These are tied with the knots outside to alleviate tension and provide accurate placement of the remaining sutures. Posterior sutures can be difficult to place and tie with the knots on the outside, and two or three sutures can be conveniently placed with the knots on the inside. The knots create minimal bronchial obstruction and are readily absorbed with little reaction. The remaining sutures are placed to achieve cartilage-to-cartilage approximation with the knots on the outside. Sutures are placed at equal depth and at an equal distance apart, as oblique placement of sutures to achieve a decrease in lumen disparity results in bronchial distortion or tearing of the cartilage due to excessive tension. Polydioxane is the preferred suture of other surgeons.43
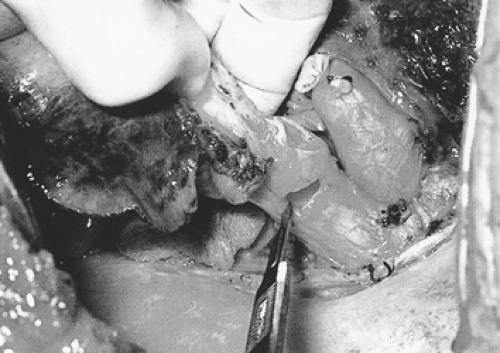 Figure 29-4. A knife transects the left mainstem bronchus for a left-lower-lobe sleeve lobectomy. The left-upper-lobe bronchus has already been cut. |
Lumen disparity is corrected by stretching the membrane of the smaller bronchus and crimping the membrane of the larger bronchus. I prefer not to resolve lumen disparity by resecting a small wedge of the cartilaginous part of the proximal bronchus to reduce its diameter as this technique creates more opportunity for bronchial dehiscence and necrosis. The final four or five sutures are not tied until all have been placed, to ensure proper
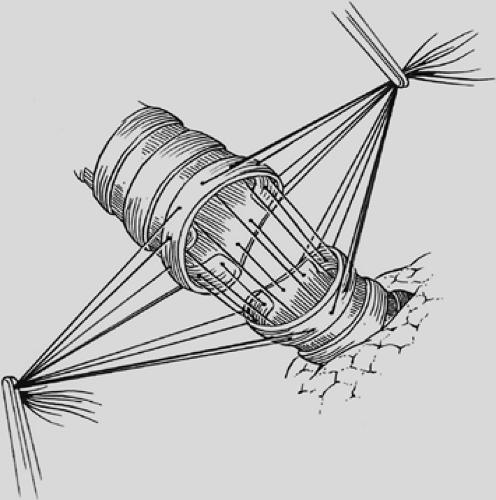
visualization of their placement. The anastomosis is covered with a pleural flap or with a pedicled anterior mediastinal fat pad or with the previously constructed intercostal muscle flap. This tissue separates the pulmonary artery from the bronchial anastomosis and minimizes the complication of a bronchial arterial fistula. Tissue coverage can also minimize the complication of a small bronchial disruption (Fig. 29-6). Another preferred anastomotic technique is to place all the sutures circumferentially before they are tied (Fig. 29-7).
visualization of their placement. The anastomosis is covered with a pleural flap or with a pedicled anterior mediastinal fat pad or with the previously constructed intercostal muscle flap. This tissue separates the pulmonary artery from the bronchial anastomosis and minimizes the complication of a bronchial arterial fistula. Tissue coverage can also minimize the complication of a small bronchial disruption (Fig. 29-6). Another preferred anastomotic technique is to place all the sutures circumferentially before they are tied (Fig. 29-7).
 Figure 29-5. Right-upper-sleeve lobectomy specimen. Frozen section is accomplished on bronchial resection margins. |
Kutlu and Goldstraw20 described the use of a continuous suture of 3-0 polypropylene in 66 sleeve lobectomies. Results were compatible with others reporting interrupted suture techniques. A smaller distal bronchus can also be telescoped into the larger proximal lumen as described by Takeda.35 This technique can be readily applied when performing left lower lobe sleeve lobectomies and reimplantation of the middle lobe or upper lobe into the mainstem bronchus. Hollaus14 described the telescoped anastomosis in 15 patients with significant bronchial lumen disparity; there was one anastomotic dehiscence with no long-term stenosis. Following the completion of the anastomosis, the lung is inflated and the saline covered bronchus is observed for any evidence of air leak at 20 to 30 cm of airway pressure. Small needle hole air leaks are of no concern, but an air leak between the edges of the bronchi may require an additional suture of 5-0 polyglycolic suture. The lung should inflate and deflate readily, indicating a widely patent anastomosis.
Failure of the lung to deflate can indicate anastomotic distortion or narrowing.
Flexible bronchoscopy is necessary to visualize the anastomosis and clear the distal airway of any bloody secretions. If the anastomosis is distorted or significantly narrowed, it may be necessary to remove some sutures and reconfigure a portion of the anastomosis. It is usually not necessary to redo the entire anastomosis.
Angioplasty is readily accomplished in conjunction with sleeve lobectomy. Rendina30 described pulmonary artery reconstruction in 40 patients with bronchial sleeve lobectomy or bilobectomy. Linear resection and closure of the main artery can be done, but the surgeon must be certain that the arterial lumen is not significantly narrowed (Fig. 29-8). It is usually more appropriate to carry out a patch angioplasty with a piece of pericardium or bovine pericardium to ensure lumen patency (Fig. 29-9). A sleeve resection of the pulmonary artery can be done in conjunction with the sleeve lobectomy, and the arterial anastomosis is accomplished with fine monofilament suture in a continuous fashion (Fig. 29-10). The arterial anastomosis is done after the bronchial anastomosis has been completed to minimize retraction and handling of the vascular anastomosis, and the transected artery permits excellent exposure for the bronchial anastomosis. On occasion, an extended arterial defect does not permit reanastomosis, and a graft of autologous pericardium is constructed30 (Fig. 29-11). Prosthetic material can also be utilized for the pulmonary artery conduit. Heparin (5,000 U) is given prior to a sleeve resection of the pulmonary artery, and subcutaneous heparin is maintained for 7 to 10 postoperative days. Long-term anticoagulation is not necessary unless a conduit has been placed and then anticoagulation is maintained for 3 months.
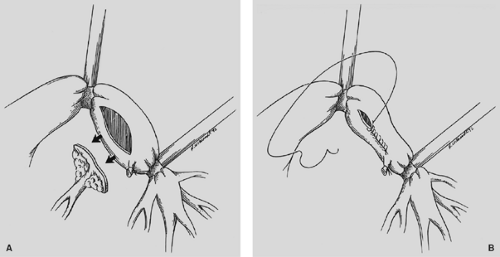 Figure 29-8. A: Elliptical portion of pulmonary artery is resected. B: Linear closure without lumen compromise of pulmonary artery. |
Postoperative Management and Complications
Many patients with lung cancer have comorbidities, which can influence the postoperative complication rate. Hollaus14 evaluated the risk factors for the development of postoperative
complications in 108 patients undergoing bronchoplastic procedures for bronchial malignancy. Two pulmonary risk factors coupled with coronary artery disease were predictive for septic complications. Peripheral artery disease, extended resections, and chronic obstructive pulmonary disease were predictive for aseptic complications. Preoperative efforts to minimize these risk factors should always be considered and undertaken.
complications in 108 patients undergoing bronchoplastic procedures for bronchial malignancy. Two pulmonary risk factors coupled with coronary artery disease were predictive for septic complications. Peripheral artery disease, extended resections, and chronic obstructive pulmonary disease were predictive for aseptic complications. Preoperative efforts to minimize these risk factors should always be considered and undertaken.
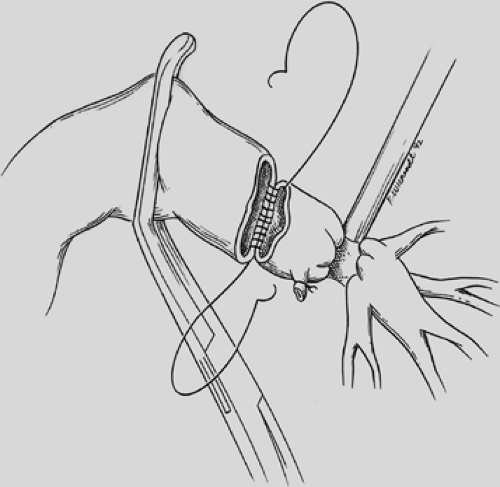 Figure 29-10. Sleeve resection of pulmonary artery. (From Faber LP. Sleeve resections for lung cancer. Semin Thorac Cardiovasc Surg 1993;5:238–248.) |
In a survey of 1,125 sleeve lobectomies performed in various centers, Tedder36 noted that pneumonia and atelectasis occurred in approximately 6.7% and 5.4% of patients, respectively. This relates to an accumulation of secretions and blood at the anastomosis due to mucosal damage and loss of ciliary function. Bronchoscopy in the operating room immediately after completion of the anastomosis can minimize this problem.
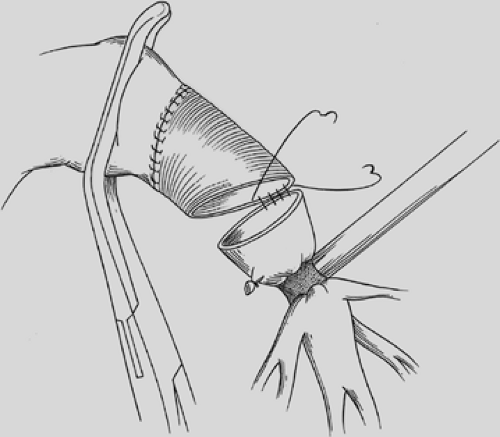 Figure 29-11. Conduit reconstruction of pulmonary artery after tumor resection. A proximal vascular clamp does not compromise the lumen for anastomosis. |
Risk factors that can have a significant effect on the development of postoperative complications include an FEV1 under 40%, increased pulmonary artery pressure, coronary artery disease, current smoking, right sided resections, and bilobectomy. Age, gender, induction therapy, vascular sleeve resection, and a positive bronchial microscopic margin appear to have no significant effect on the risk of postoperative complications.43
The clinical indication for postoperative bronchoscopy includes a persistent coarse wheeze at the anastomosis, continuing loss of volume noted on the chest radiograph, lobar consolidation, or a persistent air leak at 7 days postoperatively. Excessive secretions can be managed by minitracheostomy, if daily bronchoscopy is necessary. If the clinical course of the patient is satisfactory and the residual lung is well expanded, routine postoperative bronchoscopy prior to discharge is not done. Follow-up bronchoscopy to inspect the anastomosis at 3 months is at the discretion of the surgeon. A persistent air leak at 7 to 10 days postoperatively may indicate partial anastomotic dehiscence, and the anastomosis should be inspected with the flexible bronchoscope. Bronchial necrosis is indicated by a gray–white discoloration of the mucosa; a to-and-fro motion of secretions through a partial dehiscence can also be identified. A dehiscence of 4 to 5 mm can be observed and treated conservatively with prolonged chest tube drainage. This is particularly true if tissue has been placed over the bronchial anastomosis. The development of an airspace and increasing volume of air leak indicate further dehiscence requiring completion pneumonectomy. An anastomotic dehiscence greater than 10 mm also requires consideration of completion pneumonectomy. The surgeon must proceed with caution if suture anastomotic reapproximation is done and fresh tissue coverage with a muscle flap is necessary. Careful judgment is required because the patient must be able to tolerate the pneumonectomy procedure. Tedder36 reported a 3.5% incidence of bronchopleural fistula (42 in 1,186 patients). Completion pneumonectomy carries a mortality rate of 15% to 20%, as reported by Kawahara17 and Van Schil.39 Schirren and colleagues33 reported a 7.4% incidence of bronchopleural fistula in their series of complex bronchoplastic procedures in association with many angioplastic repairs. Tronc38 reported a fistula rate of 1.1% (2 of 184) in patients undergoing sleeve lobectomy for lung cancer. Both patients were managed conservatively, and this excellent result relates to precise preoperative evaluation and technical experience. Kawahara17 reported a 5.6% fistula rate (6 of 112 patients). However, in 24 patients with anastomotic complications, 19 were stage IIIA or IIIB, and pulmonary artery angioplasty was performed in 37 patients, indicating the advanced stage of disease in these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree