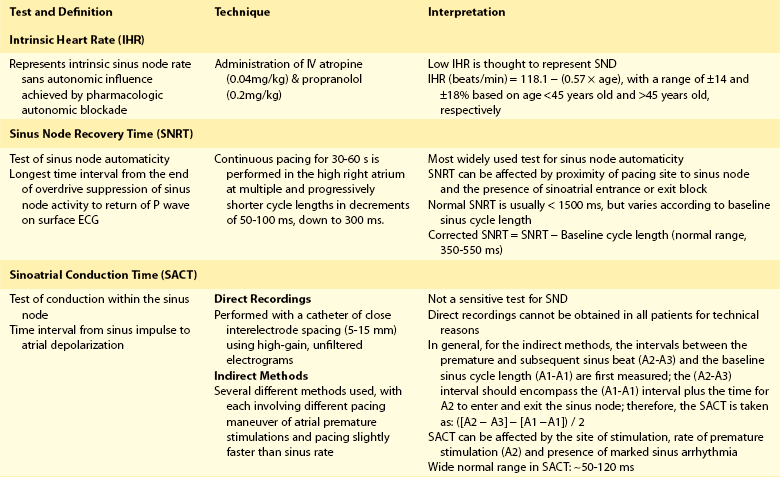
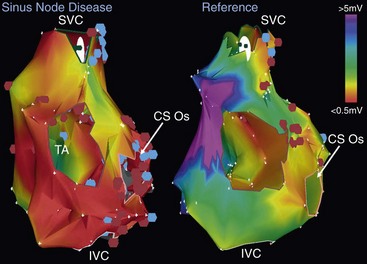
72 Although the sinus node has been recognized as the primary cardiac pacemaker for more than a century, our understanding of its complex anatomy, molecular construct, and pacemaking mechanisms remains incomplete.1 The cell biology of cardiac impulse initiation and propagation in regard to the sinus node are detailed in Chapters 25 and 26. This chapter explores the unique anatomic and physiological features of the sinus node, electrophysiological abnormalities in sinus node disease (SND), the genetic basis for familial SND, and the factors predisposing to sinus node remodeling. The sinus node consists of nodal cells packed in a dense connective tissue matrix. Detailed histologic examinations revealed that nodal cells are also found intermingled with ordinary atrial myocytes at the nodal periphery. In addition, nodal extensions are also seen extending toward the superior vena cava, subepicardium, and terminal crest.2 Recently, immunohistochemical analysis showed a new discrete paranodal area with loosely packed myocytes that is not contiguous, but in close proximity to the sinus node (Figure 72-1, A).3 The molecular construct of this paranodal area reflects a mixture of the sinus node and atrial cells with unique ion channel make-up, reduced connexin43 and atrial natriuretic peptide expression. The relatively depolarized state of this paranodal area compared with the right atrium could therefore facilitate conduction from the sinus node. Further insight regarding the sinus node can also be derived from an anatomic model of the sinus node complex based on high-resolution optical mapping studies (see Figure 72-1, B). The sinus node appears to be insulated by conduction barriers consisting of coronary arteries and fibrous tissues, with specialized preferential conduction pathways that carry the electrical impulse to the surrounding atrium.4 Indeed, a high-density noncontact mapping study had also demonstrated these preferential pathways of conduction between the sinus node and the atrial exit sites (see Figure 72-1, C), although none of these insulated tracts have been documented histologically.2,5 Figure 72-1 The sinus node revisited. A, This Masson trichrome stain of the human sinus node shows the newly described paranodal area, which contains loosely packed myocytes consisting of a unique K+ ion channel composition that is intermediate between that of the sinus nodal and atrial cells. Note that his paranodal area is not contiguous to the sinus node. B, An anatomic model of the sinus node complex based on recent high-resolution optical mapping studies. The sinus node appears to be insulated by conduction barriers consisting of coronary arteries and fibrous tissues, with specialized preferential conduction pathways that carry the electrical impulse to the surrounding atrium. C, A composite electroanatomic map of the human right atrium. It demonstrates recent evidence for preferential pathway of conduction in the sinus node from earliest activation (1) via a preferential pathway (2, 3) to sinus breakout (4) before conducting to the remaining atrium. LA, left atrium; SVC, superior vena cava; BB, Bachmann bundle; CT, crista terminalis; RAA, right atrial appendage; PV, pulmonary vein; IAS, interatrial septum; FP, fat pad; IVC, inferior vena cava; CS, coronary sinus; RV, right ventricle. (A, From Chandler NJ, Greener ID, Tellez JO, et al: Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker, Circulation 119:1562–1575, 2009. B, From Fedorov VV, Glukhov AV, Chang R: Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias, Am J Physiol Heart Circ Physiol 302:H1773–H1783, 2012. C, From Stiles MK, Brooks AG, Roberts-Thomson KC, et al: High-density mapping of the sinus node in humans: role of preferential pathways and the effect of remodeling. J Cardiovasc Electrophysiol 21:532–539, 2010.) In addition, both the sites of sinus activation and sinoatrial exit sites have been shown to be highly variable in keeping with a prior epicardial mapping study, demonstrating a widely distributed atrial pacemaker complex centered about the long axis of the sulcus terminalis.5 Specifically, the beat-to-beat variation in the site of sinus activation ranged 0 to 41 mm from the superior vena cava and right atrial junction, and the variation in sinoatrial exit site ranged 0 to 33 mm. It is also known that changes in heart rate, neural, and hormonal factors can cause a shift in both of these pacemaking and exit sites. For example, sympathetic stimulation results in a superior shift of the dominant pacemaking site, and an increased in heart rate while vagal activation leads to a slower but more caudal pacemaking focus.6 The multicentricity of the sinus node complex therefore accounts for the high incidence of spontaneous P wave variations seen in normal individuals. It is also likely that the aforementioned paranodal area and preferential conduction pathways are contributors to the multicentricity properties of the sinus node. Table 72-1 presents a summary of various conventional electrophysiological measures of sinus node function. These measures of sinus node function in patients with SND can reveal a low intrinsic heart rate, prolonged sinus node recovery time and sinoatrial conduction time. Of note, it is well established that these tests have their own limitations with wide variations in their normal range. However, electroanatomic mapping has provided further insight regarding the electrophysiological changes in SND with attenuation of the multicentricity property of the sinus node complex, resulting in a more caudal and unicentric pacemaking site close to the region with largest voltage at the crista terminalis.7 In addition, multiple early studies have shown evidence of atrophy and increased fibrous tissues in the sinus node of patients with sick sinus syndrome.8 Importantly, significant atrial abnormalities have been described in patients with sinus node disease exemplifying an atrial myopathy. These include left atrial enlargement, prolonged P wave duration, increased refractoriness, conduction slowing with increased double potentials and fractionated atrial electrograms, as well as structural changes with extensive regions of low voltage and scar (Figure 72-2).7 Recent advancement in noninvasive imaging of the atria using late gadolinium-enhanced magnetic resonance imaging has also associated increased left atrial fibrosis in patients with significant SND requiring pacemaker implantation following atrial fibrillation ablation.9 Indeed, these changes constitute a substrate that can promote circuitous atrial propagation and perpetuation of atrial arrhythmia and could in part account for the higher atrial fibrillation recurrence rates in patients with SND following pulmonary vein isolation.10 This increased vulnerability to atrial fibrillation has also been shown experimentally in a canine model of SND.11 Furthermore, echocardiographic evaluation of atrial mechanical function has also demonstrated evidence of atrial mechanical dyssynchrony in SND, with lower mitral inflow A velocity and prolonged interatrial dyssynchrony found to be independent predictors for atrial fibrillation.12 Figure 72-2 Electroanatomic remodeling of the atria in sinus node disease: evidence of a diffuse atrial myopathy. The figure demonstrates bipolar voltage maps of the right atrium in a representative patient with sinus node disease (left) and an age-matched reference patient. Both atria are oriented in the left anterior oblique projections and have standardized voltage settings. The patient with sinus node disease displays larger atria, significantly greater regions of low voltage, and areas of complex electrograms (double potentials in blue and fractionated electrograms in pink). These figures demonstrate the presence of a diffuse atrial myopathy in patients with sinus node disease. Mutations in the SCN5A gene, which encodes the α-subunit of the cardiac sodium channel (Nav1.5), have been associated with SND and several heart rhythm disorders, including Brugada syndrome, long QT syndrome type 3, increased susceptibility to atrial fibrillation, and progressive cardiac conduction defect.13 To date, at least 20 mutations in the SCN5A gene have been identified as leading to both loss and gain of function of the Nav1.5.14,15
Sinus Node Abnormalities
The Sinus Node
Sinus Node Disease
Electrophysiological and Structural Abnormalities
Atrial Abnormalities in Sinus Node Disease
Familial Sick Node Disease
SCN5A Mutations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sinus Node Abnormalities