Chapter 5 Single-Photon Emission Computed Tomography Artifacts
TECHNICAL ARTIFACTS
Flood Field Nonuniformity
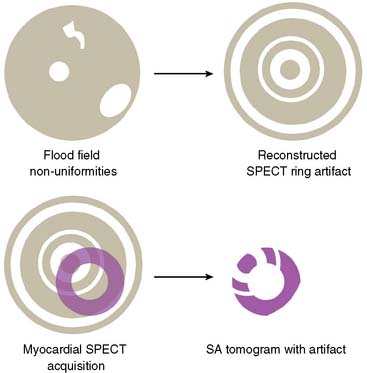
Flood field nonuniformity will result in “ring” artifacts in reconstructed SPECT images. These relatively photon-deficient rings may be apparent in tomographic slices and in severe cases may also appear in polar coordinate maps (Fig. 5-1). Because patients are frequently positioned differently within the camera field for rest and stress scans, flood field artifacts may occur in differents of the myocardium, mimicking reversible or partially reversible defects. Therefore, routine acquisition and inspection of intrinsic and extrinsic flood fields acquired according to vendor recommendations are absolutely critical to avoid such artifacts. Flood fields are routinely acquired the first thing in the morning each working day. However, if ring artifacts appear in clinical SPECT images, it may be necessary to reacquire flood field images in the middle of the day.
Center of Rotation and Camera-Held Alignment Errors
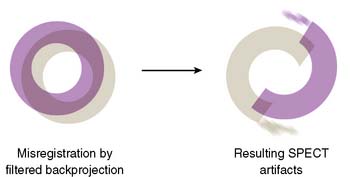
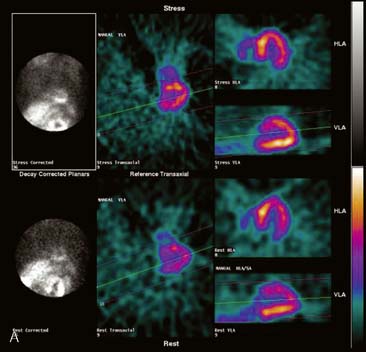
If the camera center of rotation (COR) is incorrect, filtered backprojection during SPECT reconstruction will result in image misregistration and apparent misalignment of the myocardial walls (Fig. 5-2). Technically, this is similar to the error created by cardiac motion. However, unlike motion artifacts, those due to COR error are usually more systematic and predictable. The severity of the apparent defect is directly proportional to the magnitude of the COR error. An error similar to that produced by the wrong center of rotation is produced when the detector is not aligned perpendicular to the radius of rotation.
Errors in Selecting Oblique Cardiac Axes and Subsequent Polar Map Reconstruction
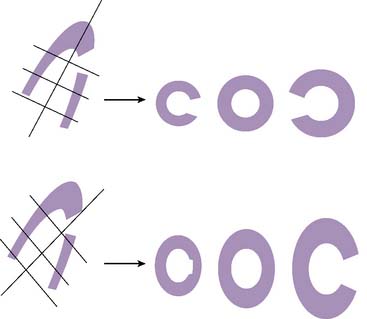
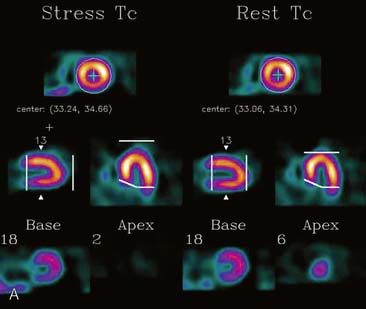
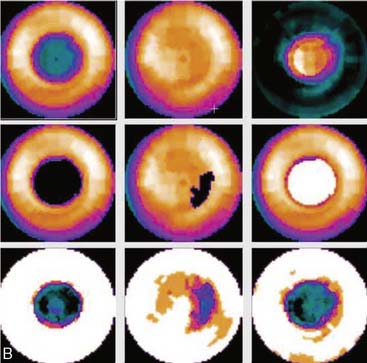
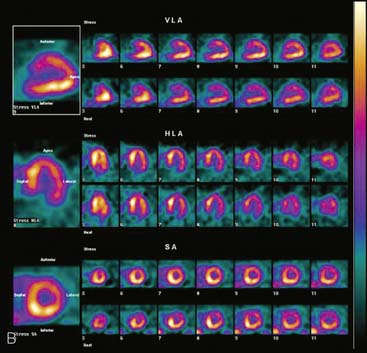
If the long axis of the left ventricle (LV) is defined incorrectly on either the transaxial or midventricular vertical long-axis slice, the geometry of the heart in subsequently reconstructed orthogonal tomographic slices can be distorted. Consequently, the apparent regional count density can be altered in polar maps, resulting in apparent perfusion defects. These may be accentuated by quantitative analyses in which patient data are compared to normal files (Fig. 5-3). In polar map reconstruction, such errors most often occur in basal myocardial regions at the periphery of the bull’s-eye plot, owing to foreshortening of one of the myocardial walls (Fig. 5-4). Also, the apex, which often demonstrates physiologic thinning and decreased count density, is displaced from the exact center of the polar plot. The displaced apex may also consequently result in an artifact. Misregistration of perfusion defects in short-axis slices and polar maps is not infrequently encountered in patients with sizable severe infarcts.
Selection of Apex and Base for Polar Map Reconstruction
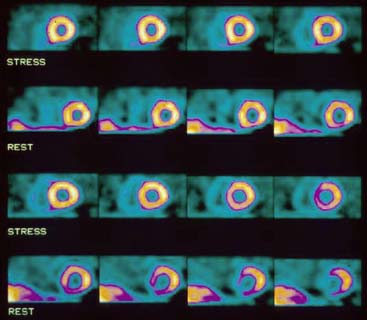
Accurate and reproducible selection of the apex and base of the LV myocardium is necessary in stress and rest images. Positioning limits for slice selection that lie too far apically or basally will result in apparent perfusion defects (Fig. 5-5). In contrast, positioning slice limits too tightly, so that they do not encompass the entire heart, will potentially result in underestimation of the size and extent of a defect. Likewise, in regions of normal myocardium, maximal myocardial count density may not be correctly sampled.
Arrhythmias and Gating Errors
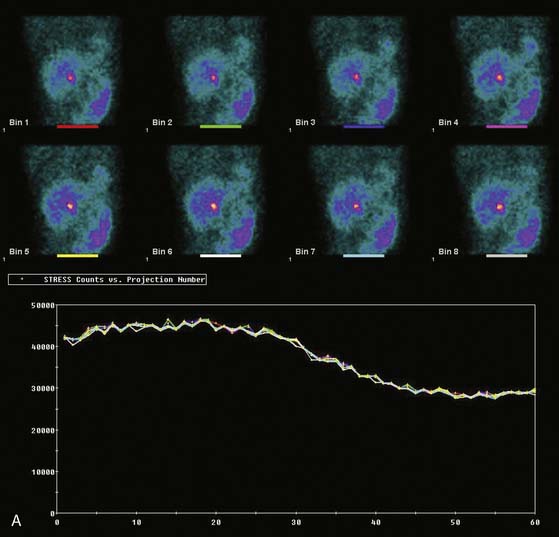
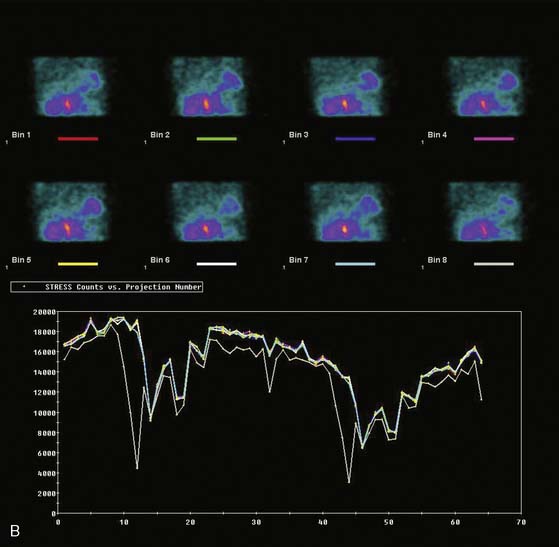
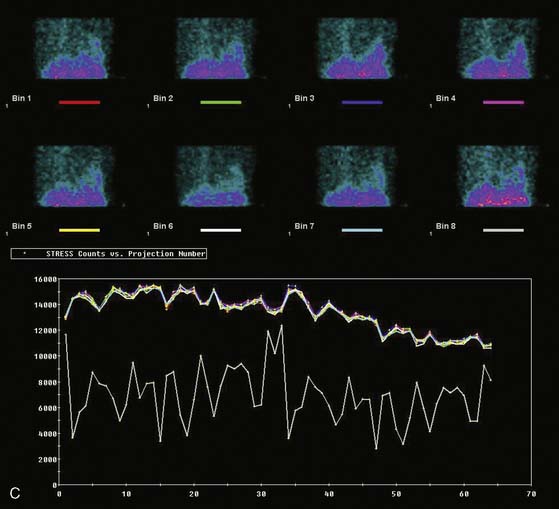
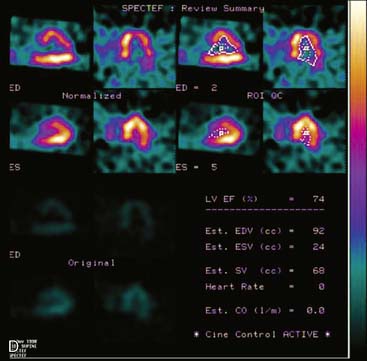
Displays of cardiac image count density from individual planar projection images is now possible using some commercially available software programs (Fig. 5-6). Errors in LV volume and ejection fraction may result from gating errors, but these are beyond the scope of this chapter.1
PATIENT-RELATED ARTIFACTS
Soft-Tissue Attenuation (See Chapters 6 and 7)
The location of the attenuation artifact depends on the position of the soft-tissue attenuator in relation to the left ventricle. The severity of the artifact depends on the size and density of the attenuator in relation to adjacent tissue. Within the resultant SPECT image, the artifact may appear as a fixed or reversible defect or may mimic “reverse redistribution,” depending on whether the attenuator is in a constant or variable position in stress and delayed image acquisitions. The severity of the attenuation artifact also depends on the energy of the incident photon. Attenuation artifacts for technetium (99mTc)-labeled myocardial perfusion agents will be somewhat less marked than for thallium (201Tl).3 However, for either isotope, evaluation of regional wall motion is helpful in differentiating attenuation artifacts from myocardial scarring as a source of fixed perfusion defects.4–6
Breast Attenuation
Because breasts vary in size, position, configuration, and density, breast attenuation artifacts are extremely variable in appearance. In addressing the characteristics of breast attenuation, it is always necessary to consider the position and configuration of the breasts with the patient in the supine position, because patients are imaged in this position with most commercially available SPECT systems. In women of average body habitus, the left breast overlies the anterolateral wall of the heart. In women with large, pendulous breasts, the breasts lie adjacent to the lateral chest wall and more often result in a lateral attenuation artifact. In some women with very large, pendulous breasts, the attenuation artifact may create apparent inferior or inferolateral perfusion defects. In women with very large breasts, the breast tissue may overlie the entire LV. In this instance, the resulting attenuation artifact may be diffuse and less discrete or may primarily involve the apex. In addition to breast position, the caudal angulation of the heart within the thorax will affect the appearance of the attenuation artifact.2–3 Thus, in summary, the severity of breast attenuation artifacts is not necessarily directly proportional to breast size or chest circumference but may vary considerably according to the position, configuration, and density of the breast, body habitus of the patient, and orientation of the heart within the thorax. Additionally, when women are imaged upright or in a semi-upright, “reclining” position, the breasts are usually more pendulous than in the supine position. Therefore, apical and inferior breast attenuation artifacts are more frequent when women are imaged in these positions.
Attenuation correction for SPECT is now commercially available but is still a topic of intense interest and research. The methods for accomplishing it have used either scatter correction or attenuation correction using a separate radionuclide or x-ray transmission image. This approach has been demonstrated to significantly reduce breast and other attenuation artifacts in phantom and patient studies.7–18 However, whereas attenuation correction may minimize attenuation artifacts created by overlying breast tissue, breast artifacts are frequently not totally eliminated.
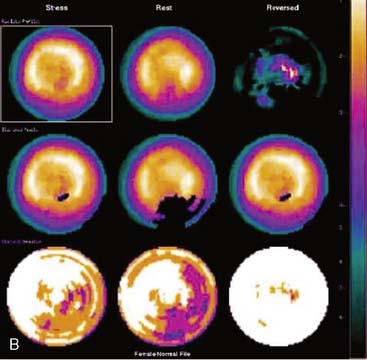
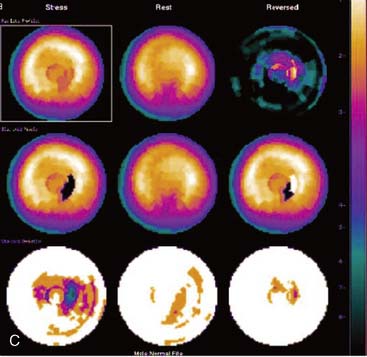
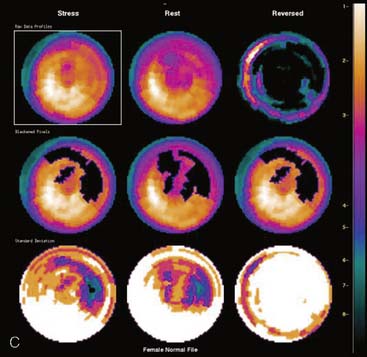
Certain breast attenuation artifacts are unique to quantitative analysis in cardiac SPECT. The most common is an apparent inferior perfusion defect incorrectly identified by quantitative analysis in women who have had a left mastectomy. After removal of the breast, there is little or no anterior soft-tissue attenuation. In such cases, the anterior and inferior myocardial count densities are nearly the same as they are for males. Since the normal female file anticipates that the inferior wall will be more intense than the anterior wall, and in the patient study the inferior wall has an identical or lower intensity than the anterior wall, the inferior wall will be identified as abnormal (Fig. 5-7). This quantitative error can be circumvented by comparison of the patient’s data to the normal male file instead. Similar inferior artifacts have been observed in quantitative plots in women with very small breasts, and it might be argued that data from such patients should be compared to normal male limits. However, small breasts may be quite dense, and the assumption that they do not produce photon attenuation may be incorrect. Therefore, in most laboratories, the normal male file is used routinely only for women who have undergone left mastectomy.
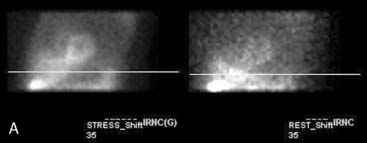
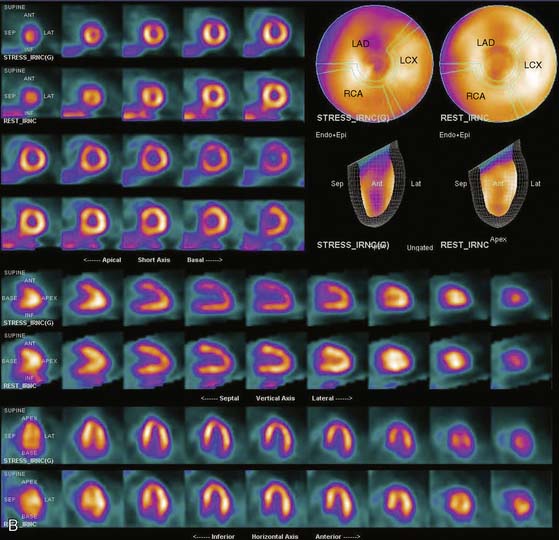
The position of the breast may vary from stress to rest if a woman wears different clothing at the time of the two SPECT acquisitions. Breast position varies considerably with a bra on and off. The position of the breast may also vary depending upon the degree of elevation of the left arm, which is preferably positioned above the head for a SPECT acquisition. A shifting breast attenuation artifact can mimic a reversible perfusion defect (i.e., ischemia). For example, if during stress SPECT acquisition, the breast lies high over the anterior chest wall, an anterior attenuation artifact may result. However, if during the resting acquisition, the breast lies more laterally and inferiorly, the artifact will involve the inferolateral wall of the LV. The resulting attenuation artifacts are therefore different in the stress and rest images, so artifactual defect reversibility as well as artifactual “reverse distribution” may result. In this example, the anterior defect will appear to be reversible, whereas there will appear to be “reverse distribution” in the inferolateral wall (Fig. 5-8).
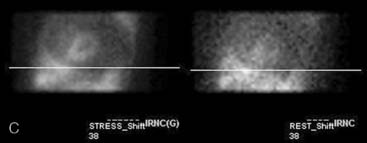
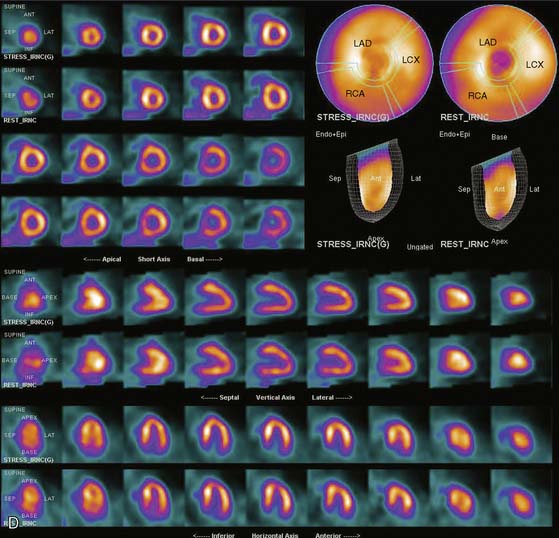
Gated myocardial perfusion SPECT is helpful in differentiating breast attenuation artifacts from scar, since artifacts will demonstrate normal wall motion and wall thickening, whereas infarcts, if they are transmural and sizable, will be hypokinetic with decreased wall thickening (Fig. 5-9).4,5 However, patients with nontransmural myocardial infarctions might have normal wall motion, depending on the thickness of the infarct zone. For artifacts created by a breast that shifts in position from stress to rest, gating is less helpful to differentiate artifact from ischemia as a cause of the reversible or partially reversible defect.
Lateral Chest-Wall Fat Attenuation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree