Secondary Tumors of the Lung
Mark S. Allen
Joe B. Putnam Jr.
Pulmonary metastases represent a particular manifestation of systemic metastases from primary malignant tumors. Although primary tumors can be locally controlled with surgery or irradiation, the selection of therapy for systemic metastases requires systemic-type therapies such as chemotherapy or other, targeted therapy. Metastases are commonly treated with chemotherapy as an initial modality. Local control modalities such as radiation therapy, or even surgery, may be used to treat or palliate the local symptoms resulting from metastases, particularly bony metastasis causing pain. Although metastases often represent systemic and uncontrolled tumor growth, which may herald rapid disease progression, patients with metastases isolated within the lung may have a more favorable tumor biology. These patients are more amenable to local treatment options, or combinations of local and systemic treatment options, than are patients with multiorgan metastases. Isolated pulmonary metastases should not be viewed as untreatable. Patients who have complete resection of all metastases have a longer survival than patients who have pulmonary metastases left behind. Long-term survival, >5 years, may be expected in about 30% of all patients with resectable pulmonary metastases.
Most patients with metastatic disease to the lung, however, have unresectable metastases. Only a small minority of these patients are amenable to complete resection of pulmonary metastasis (i.e., physical removal or ablation of all abnormalities that can be visualized or palpated). Selection of therapy for patients with pulmonary metastases isolated to the lung requires multidisciplinary evaluation in all but the simplest of situations. Isolated solitary metastasis with a prolonged disease-free interval (>12 months) may be resected with good results. Combinations of chemotherapy and surgery may be considered and may offer more patients the potential for optimal local and systemic control of their disease process. Enhancement of survival will require improved local control, systemic therapies, or regional drug delivery to the lungs.
Historical Perspective
Early attempts of resection of pulmonary metastases have been described by Meade164 and Martini and McCormack.156 Weinlechner265 and Kronlein126 reported resection of pulmonary metastasis (as an incidental procedure) while resecting a primary chest wall tumor. Resection of a pulmonary metastasis as a planned procedure was described by Divis49 and Torek.124 Barney and Churchill14 reported one of the first long-term survivors of pulmonary metastasectomy after resection of a metastasis from a patient with hypernephroma (metastatic renal cell carcinoma). After nephrectomy for local control of the primary tumor, the patient underwent resection of the metastasis. The patient survived for 23 years and died from unrelated causes. Alexander and Haight3 reviewed the first large series (25 patients) of those who had resection of metastases from carcinoma and sarcoma. They concluded that patients who would withstand the resection and in whom no other metastases were evident could undergo resection. Mannix152 described, for the first time, resection of multiple pulmonary metastases from a patient with osteochondroma of the tibia. Only one nodule was identified on the preoperative chest radiograph. Few attempts were made at multiple or repetitive resections for pulmonary metastases until Martini and associates157 described the value of resecting multiple metastases and the associated survival advantage of multiple resections (multiple sequential operations) for treatment of osteogenic sarcoma. Selection criteria have been proposed by many; however, Putnam and Roth197 noted that unresectability may be the sole exclusion criterion. Selection for resection is otherwise subjective and individualized to the patient. In the past 20 years, resections of solitary and multiple pulmonary metastases from numerous primary neoplasms have been performed with long-term survival in 20% to 40% of patients, as shown by Pastorino and colleagues.183
Autopsy studies have demonstrated that about one-third of patients with cancer die with pulmonary metastases and that a small percentage die with metastases confined solely to the lungs. Metastases from osteogenic and soft tissue sarcomas commonly occur only in the lungs, as shown by Potter and colleagues.196 Less commonly, patients with other solid organ neoplasms from melanoma, breast, or colon have isolated pulmonary metastases, but these metastases may represent favorable tumor biology and a treatable subset of such patients. In the absence of extrathoracic metastases, patients with isolated and resectable pulmonary metastases should undergo complete resection in an attempt to prolong survival and possibly cure their disease. Even in the presence of extrathoracic metastases, selected individual patients with complete resection may have a survival advantage. Limitations on the number of metastases resected with benefit have not as yet been determined; however, the greater the number of radiographically identified metastases before resection, or the larger the number palpated in
the operating room, the greater likelihood that micrometastases exist and that the lesions are unresectable. Early and potentially aggressive recurrence is likely. Multidisciplinary evaluation and selection of effective systemic therapy may theoretically treat micrometastatic disease, thus increasing the postdiagnosis of metastases survival beyond that of immediate resection. Still, local control of a rapidly enlarging solitary metastasis may be needed. Progression of multiple metastases will inexorably reduce pulmonary reserve.
the operating room, the greater likelihood that micrometastases exist and that the lesions are unresectable. Early and potentially aggressive recurrence is likely. Multidisciplinary evaluation and selection of effective systemic therapy may theoretically treat micrometastatic disease, thus increasing the postdiagnosis of metastases survival beyond that of immediate resection. Still, local control of a rapidly enlarging solitary metastasis may be needed. Progression of multiple metastases will inexorably reduce pulmonary reserve.
Pathology
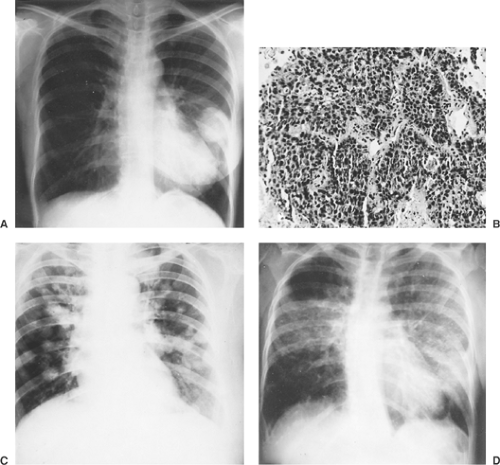
Malignant tumors may metastasize by hematogenous, lymphatic, and aerogenous routes or by direct invasion. Underlying tumor biology and host resistance determine mechanisms of spread, location of metastases, and extent of growth. Hematogenous metastases are most frequently found in the capillary beds of the lung, liver, brain, and bone. Clumps of tumor cells that metastasize to the lung parenchyma may be trapped or preferentially adhere to the underlying capillary endothelium. Most of these tumor emboli die; however, some may permeate the endothelium and grow. Tumor cells may travel by lymphatics and occupy a discrete position within the lung parenchyma, or they may diffusely involve the entire lung (e.g., lymphangitic spread of breast carcinoma or other metastatic adenocarcinomas) (Fig. 126-1). Pulmonary metastases may metastasize to other organs. Depending on the primary histology (usually related to adenocarcinoma or squamous cell carcinoma primary tumors), metastases can develop in draining pulmonary lobar, hilar, or mediastinal nodes. Direct invasion of metastases into other structures may occur as the metastasis grows. Resection of the pulmonary metastasis and the contiguous structure is recommended. Putnam and associates200 noted that extended resection may achieve local control and survival benefit if complete resection of metastases can be achieved with negative margins. Finally, aerogenous spread of tumor from one site within the tracheobronchial tree to another is rare if it occurs at all.
Symptoms
Symptoms rarely occur from pulmonary metastases. Therefore diagnosis of metastases is routinely made on radiographic imaging after primary tumor resection. Palliation for pain is rarely needed because the parietal pleura is infrequently involved by parenchymal metastases. A distinction must be made between pleural-based and parenchymal-based metastases before resection. Few (<5%) patients with metastases present with symptoms of dyspnea, pain, cough, or hemoptysis. Bocklage and colleagues25 noted that patients with metastases from angiosarcoma would present with these symptoms, having lasted from a few weeks to months. Rarely, patients with peripheral sarcomatous metastases may develop pneumothorax from disruption of the peripheral pulmonary parenchyma. Srinivas and Varadhachary238 have suggested that patients with a primary malignancy and a pneumothorax should be evaluated for lung metastases.
Diagnosis
Pulmonary metastases may appear as solitary or multiple nodules and as well-circumscribed or diffuse opacities; they may be miliary or massive in appearance, as described by Snyder and Pugatch.234 Still, the radiographic findings of patients with pulmonary nodules may be nonspecific and represent a wide spectrum of benign or malignant processes. The surgeon must consider other, more locally related diagnoses such as histoplasmosis, tuberculosis, or other malignant diseases such as lung cancer in these patients. There are no pathognomonic radiographic criteria for metastatic disease. However, multiple, small, well-circumscribed peripheral nodules in the patient with a known primary malignancy are very likely to be metastatic disease.
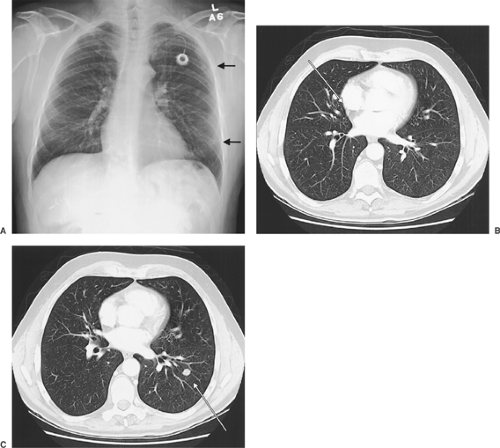
Chest radiographs are commonly obtained after primary tumor resection because they may demonstrate pulmonary parenchymal changes consistent with metastases (Fig. 126-2A). Routine chest radiographs represent an effective means of screening patients for pulmonary metastases. The chest radiograph itself is an effective screening tool. Fleming and colleagues64 found that <1% of patients with T1 primary extremity soft tissue sarcomas had pulmonary metastases detectable on chest radiographs (with selective use of chest computed tomography [CT]). These authors found that routine chest CT for all patients with extremity T1 soft tissue sarcoma was not an efficient means of detecting occult metastases in this patient population. In one study, Ren and colleagues205 noted that chest radiographs identified only 48% of patients with metastases. Lien and coworkers146 showed that about half of patients with nonseminomatous testicular tumors had negative chest radiographs but abnormalities identified on CT scans.
Patients with known metastases on chest radiographs should undergo CT to identify the precise location of the known metastases and identify other smaller, potentially occult metastases (Fig. 126-2A–C). CT will demonstrate nodules as small as 2 to 4 mm. When clinically correlated with the patient’s age, prior history of malignancy, and prior treatment, a clinical diagnosis of pulmonary metastases can be made.
Patients without evidence of metastases on chest radiograph may have metastases demonstrated by CT. Today’s high-resolution CT of the chest may achieve resolution of pulmonary abnormalities 2 to 3 mm in diameter. Metastases may appear at this size, but more commonly sequelae of infections such as granulomas or other pulmonary parenchymal changes may produce these small indeterminate lesions. In specific parts of the country, granulomatous disease from histoplasmosis is prevalent and clinical correlation with the radiographic size and number of the lesions, location, physical and radiographic characteristics, and character must be considered. Resection may provide the most direct way to evaluate histology; however, a benign etiology is more common in the general population. Margaritora and associates154 noted that helical computed tomography (HCT) of the chest was more sensitive than high-resolution CT of the chest (81% versus 75%). Sensitivity for lesions <6 mm in diameter was 48% to 62%. Margaritora and colleagues154 noted that CT of the chest achieved a sensitivity of only 48% for lesions <6 mm. More recently the use of maximum intensity projection images from HCT has been shown to facilitate recognition of small pulmonary nodules.38 In exploring patients with such findings, the surgeon must be prepared to palpate these small lesions carefully, as they may lie deep within the lung parenchyma. The authors also noted that HCT was more sensitive than high-resolution CT. Commonly, indeterminate lesions are followed for changes on sequential CT scans. If these indeterminate nodules enlarge in size on subsequent high-resolution or spiral CT, then resection or other treatment would be planned. Chest CT provides a valuable and consistent anatomic reference for preoperative assessment of the extent of resection necessary for complete removal of pulmonary metastases. Still, even with clinically resectable disease noted on preoperative imaging, thoracic exploration and thorough manual palpation are required because of the potential to underestimate the number of nodules <6 mm.
Magnetic resonance imaging (MRI) may be as sensitive as CT for identifying pulmonary metastases, but it adds little additional information, as observed by Feuerstein60 and Wyttenbach276 and their associates. The resolution of an MRI scan for pulmonary metastases is not as sharp as that of CT of the chest. A short-time inversion–recovery sequence provides the best sensitivity (82%) for individual nodules. MRI is not routinely recommended for evaluation of patients with pulmonary metastases limited to the pulmonary parenchyma, although newer techniques, such as three-dimensional (3D) volumetric interpolated breath-held whole-body MRI, as recommended by Lauenstein and colleagues,140 may be valuable in selected patients. Walker and associates262 have used MRI as a screening tool with success for extrapulmonary metastases in patients with breast carcinoma. MRI may provide complementary information to CT in planning resection for metastases involving the posterior mediastinum, neural foramina, or great vessels, as recommended by Wyttenbach and associates.276
Benign granulomatous diseases may mimic metastases; however, in patients with a prior diagnosis of malignancy, new and multiple nodules are most likely metastases. Fine-needle aspiration or thoracoscopic wedge excision may be helpful for diagnosis or staging of pulmonary nodules in high-risk patients. Clinical stage I or II primary lung carcinoma may be indistinguishable from a solitary metastasis, particularly if the original tumor was squamous cell carcinoma or adenocarcinoma. For these two histologies specifically or in patients in whom a primary non-small-cell carcinoma (NSCLC)
of the lung cannot be excluded, lobectomy and a systematic mediastinal lymph node dissection, provided the patient has sufficient pulmonary reserve, would be a procedure of choice. In patients with lymphangitic spread of cancer and dyspnea, biopsy may be required to differentiate neoplasm from infection.
of the lung cannot be excluded, lobectomy and a systematic mediastinal lymph node dissection, provided the patient has sufficient pulmonary reserve, would be a procedure of choice. In patients with lymphangitic spread of cancer and dyspnea, biopsy may be required to differentiate neoplasm from infection.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) can be used to identify patients with metastases. Its value may lie in a “negative” study as well as in identifying lesions that are “positive” or FDG-avid. Lucas and coworkers150 evaluated the results of FDG PET and chest CT in 62 patients who had been treated for pulmonary metastases (mean age, 51 years) with 15 types of soft tissue sarcoma. Local recurrence, distant recurrence, and pulmonary metastases were evaluated. The mean follow-up was 3 years. For local disease, FDG PET was 73.7% sensitive and 94.3% specific (14 true positive, 5 false negative). MRI was 88.2% sensitive and 96.0% specific. When FDG PET was used to identify lung metastases in 70 comparisons, sensitivity was 86.7%, and specificity 100% (13 true positive, 2 false negative). CT of the chest had 100% sensitivity and 96.4% specificity. Other metastases (13 patients) were identified by FDG PET. The authors concluded that FDG PET could identify local and distant recurrence of tumor and other metastases and recommended that all three methods be used in a complementary fashion to identify the extent of disease initially and during follow-up. Franzius and colleagues69 compared FDG PET with helical chest CT to detect pulmonary metastases arising from malignant bone tumors. FDG PET had a sensitivity of 0.50, a specificity of 0.98, and an accuracy of 0.87, compared with spiral chest CT of 0.75, 1.00, and 0.94, respectively. The authors concluded that helical chest CT is superior to FDG PET in detecting pulmonary metastases from primary bone tumors. Hung
and colleagues102 noted that the use of FDG PET for patients with a current cancer provided good information for regional as well as extraregional metastases. This observation was confirmed by Lonneux and colleagues,149 who observed that whole-body FDG PET was superior to conventional imaging modalities in patients being evaluated for recurrent colorectal carcinoma or, as found by Siggelkow and coworkers,229 recurrent breast carcinoma. Veronesi and associates260 noted that glucose uptake (by FDG PET) and angiogenesis were independent biological features in patients with pulmonary metastasis from the various neoplasms and that this may suggest future antiangiogenic therapies. In a review by Fortes and colleagues67 of 84 patients who underwent 106 resections, at least one nodule was PET-positive in 68%. The true-positive rate was 66.6% and the false-negative rate was 33.3% for all nodules.
and colleagues102 noted that the use of FDG PET for patients with a current cancer provided good information for regional as well as extraregional metastases. This observation was confirmed by Lonneux and colleagues,149 who observed that whole-body FDG PET was superior to conventional imaging modalities in patients being evaluated for recurrent colorectal carcinoma or, as found by Siggelkow and coworkers,229 recurrent breast carcinoma. Veronesi and associates260 noted that glucose uptake (by FDG PET) and angiogenesis were independent biological features in patients with pulmonary metastasis from the various neoplasms and that this may suggest future antiangiogenic therapies. In a review by Fortes and colleagues67 of 84 patients who underwent 106 resections, at least one nodule was PET-positive in 68%. The true-positive rate was 66.6% and the false-negative rate was 33.3% for all nodules.
The surgeon must select the radiographic imaging or scanning techniques that will provide the necessary and complete clinical information required for treatment-planning decisions. Woodard and colleagues274 have suggested several factors that may influence the surgeon’s choice of radiographic studies. These factors include (a) identifying the size, location, and characteristics of pulmonary nodules or metastases; (b) characterizing the solitary squamous cell carcinoma (or adenocarcinoma) metastasis from a primary NSCLC; (c) evaluating for extrathoracic metastatic disease (other sites of hematogenous spread, metastasis to regional lymph nodes, or other tumors); and (d) evaluating the potential for local invasion.
Metastasis or Primary Bronchial Carcinoma
Pulmonary metastases from sarcomas or other distinctive nonpulmonary neoplasms are easy to diagnose. Solitary carcinomatous metastasis from breast or colon cancers and squamous cell carcinoma metastasis from head and neck primary tumors are more difficult to distinguish from primary lung carcinoma. Patients with ≥2 pulmonary nodules can be considered to have metastases. Treatment may be similar. In tumors without a propensity for bilaterality (e.g., nonsarcomatous histology), a unilateral approach may be optimal.
Traditionally, a comparison of the primary neoplasm and the lung nodule using light microscopy has been the only method for determining origin of the lung nodule or neoplasm. Electron microscopy, as studied by Herrera and associates,92 or specific molecular or genetic characteristics may identify the origin of these neoplasms more precisely. Monoclonal antibodies may assist in discriminating between primary bronchial adenocarcinoma and colon carcinoma metastatic to the lung, as described by Ghoneim and colleagues.76 Amplified K-ras oncogene expression in a pulmonary metastasis from colon adenocarcinoma primary was noted by Slebos and coworkers233 and was also present in the primary tumor. A monoclonal antibody to identify colorectal carcinoma has been used by Flint and Lloyd65,66 in 46 patients. Cytology samples from patients with metastatic colon carcinoma and primary lung adenocarcinoma were examined; however, the monoclonal antibody was not effective in discriminating primary lung cancer from metastatic adenocarcinoma. Flow cytometry and DNA analysis have been used by Nomori177 and Salvati216 and their colleagues to describe primary carcinomas of the lung and distinguish them from metastases. Identical p53 mutations within a nonthoracic primary tumor and a lung nodule of similar histology may point to a pulmonary metastasis, as suggested by Kandioler and colleagues.115 Dissimilar p53 mutations may suggest a primary NSCLC rather than a metastasis.
To determine the nature of the new pulmonary nodules, Lefor and coworkers141 developed algorithms for patients with squamous cell carcinoma of the head and neck region who developed such nodules after treatment. Characteristics of metastases and of primary lung carcinoma were examined in an attempt to better direct subsequent therapy. Yet since the genotype of malignant tumors is unstable, a definitive molecular technique to differentiate metastases from a primary tumor is not available. Clinical judgment is useful in these circum- stances.
Treatment of Pulmonary Metastases
Most patients with pulmonary metastases have multiple sites of metastases or unresectable pleural or pulmonary metastases. In these patients, treatment is directed systemically for control of the disease and to palliate symptoms. Although radiation therapy or chemotherapy is frequently used, inconsistent response rarely leads to control or cure. Chemotherapy as initial therapy for these “systemic metastases” and resection as “salvage” may provide better results than resection alone. Where the primary tumor is controlled and metastases are confined to the lungs, resection of all visualized or palpable metastases may be considered. Complete resection of isolated pulmonary metastases is generally associated with improved survival regardless of primary histology.
Chemotherapy
Chemotherapy has not been used routinely for treatment of resectable pulmonary metastasis. However, with the exception of the patient with only one metastasis or with a few metastases and a long disease-free interval, occult micrometastases may commonly exist. For example, in sarcomas, control of the primary tumor may be achieved in various ways; however, later occurrence of pulmonary metastases from existing micrometastases at the time of control of the primary results in decreased survival compared with patients who do not have occult metastases. Even with multiple resections, complete eradication of all micrometastases may be unachievable. Use of chemotherapy or other targeted therapies to assist in the control of micrometastases may be valuable for systemic control, which may enhance the local control achieved by resection. The traditional measure of postresection survival and postresection disease-free survival may be inadequate when resection is considered as salvage after chemotherapy for pulmonary metastases. A more fitting measure of survival should include “survival from diagnosis including radiologic diagnosis of metastases.” The duration of chemotherapy, the extent of response, the histology of the primary malignancy, and the fitness of the patient all affect the timing of resection and potentially long-term outcomes.
During the past 20 years, the survival rate in patients with osteogenic sarcoma has improved from 20% to approximately
60% to 70%. Limb-sparing procedures have replaced amputation. Neoadjuvant chemotherapy with a variety of agents has been instituted. The incidence of pulmonary metastases in patients with primary osteogenic sarcoma treated with surgical resection and adjuvant chemotherapy has dramatically declined compared with treatment of the primary osteogenic sarcoma with surgery alone, as shown by Skinner,231 Goorin,82 and Pastorino181 and their coworkers. Hirota and colleagues98 have observed that newer agents are increasingly being incorporated into chemotherapeutic strategies. Nonetheless, Ferguson and colleagues58 confirmed that relapse still remains a significant problem in these patients. In their report, carboplatin as induction therapy was followed by resection and postoperative multidrug chemotherapy in 37 patients. No patient had a complete response. Patients with metastases confined to the lungs were more likely to survive than were patients with distant bone metastases. Salvage treatment with resection alone for pulmonary metastasis generates an actuarial survival rate of only about 30%. Salvage chemotherapy with resection may be effective in prolonging survival in patients who develop pulmonary metastases from osteogenic sarcoma, as described by Marina155 and Pastorino182 and their colleagues. However, more effective systemic therapies are necessary.
60% to 70%. Limb-sparing procedures have replaced amputation. Neoadjuvant chemotherapy with a variety of agents has been instituted. The incidence of pulmonary metastases in patients with primary osteogenic sarcoma treated with surgical resection and adjuvant chemotherapy has dramatically declined compared with treatment of the primary osteogenic sarcoma with surgery alone, as shown by Skinner,231 Goorin,82 and Pastorino181 and their coworkers. Hirota and colleagues98 have observed that newer agents are increasingly being incorporated into chemotherapeutic strategies. Nonetheless, Ferguson and colleagues58 confirmed that relapse still remains a significant problem in these patients. In their report, carboplatin as induction therapy was followed by resection and postoperative multidrug chemotherapy in 37 patients. No patient had a complete response. Patients with metastases confined to the lungs were more likely to survive than were patients with distant bone metastases. Salvage treatment with resection alone for pulmonary metastasis generates an actuarial survival rate of only about 30%. Salvage chemotherapy with resection may be effective in prolonging survival in patients who develop pulmonary metastases from osteogenic sarcoma, as described by Marina155 and Pastorino182 and their colleagues. However, more effective systemic therapies are necessary.
The results of preoperative chemotherapy (high-dose methotrexate, cisplatin, doxorubicin, and ifosfamide) followed by surgery and additional postoperative chemotherapy have been examined. Goorin and colleagues83 found that the combination of etoposide and high-dose ifosfamide as an induction regimen for patients with pulmonary metastasis from osteosarcoma can be effective despite significant myelosuppression, infection, and renal toxicity. Bacci and coworkers10 noted that, in 16 patients, chemotherapy was given, followed by simultaneous resection of the primary and metastatic tumors. Complete resection was accomplished in 15 patients. However, 5 patients died within a few months as a result of undetectable metastatic disease. Survival was strongly correlated with the chemotherapy effects (necrosis) in the primary tumor and in the metastases. Improved survival with combined-modality therapy (chemotherapy followed by salvage surgery) was achieved compared with historic results.
Chemotherapy alone may be insufficient. Jaffe and coworkers109 examined the role of chemotherapy in 31 patients with osteogenic sarcoma. Only 3 patients were cured with chemotherapy alone, while 4 patients underwent resection. No viable tumor was found in the resected mass. New therapies are needed for better treatments of osteogenic sarcoma. Until then, combinations of chemotherapy and local control (resection) will be needed. Glasser and associates78 noted that histologic response to chemotherapy (percentage of necrosis) was the only independent predictor of enhanced survival in a study of 279 patients with stage II osteogenic sarcoma.
Based on the effects of chemotherapy in the treatment of primary sarcoma, the effective use of such chemotherapy as planned induction therapy before resection of metastatic disease remains more elusive. Lanza and associates137 examined the response of soft tissue sarcoma metastases that were treated with chemotherapy before surgery. Patients were graded as having complete, partial, or no response or progression from the chemotherapy. Survival could not be predicted based on response to chemotherapy alone.
An optimal therapeutic strategy may be to combine systemic and local control, particularly in those patients with recurrent disease (pulmonary metastasis). Chemotherapeutic agents with activity in primary soft tissue sarcoma are limited. According to Benjamin23 and Patel185 and their colleagues, doxorubicin and ifosfamide are the two most active chemotherapeutic agents for soft tissue sarcoma and have a positive dose–response profile. Resection of pulmonary metastases after optimizing response to chemotherapy may enhance overall local and systemic control, resulting in improvements in overall and disease-free survival. This therapeutic model appears to provide a synergistic benefit over and above that which can be achieved with either surgery alone or with chemotherapy alone. Doxorubicin and ifosfamide had been used in a dose-intensive manner with resulting improved response rates, decreased time to progression, and improved survival, particularly in patients treated for higher-risk primary extremity soft tissue sarcoma. This regime for pulmonary metastasis from soft tissue sarcoma provides similar survival benefits to regimes for other malignancies. Patients with a biological response to chemotherapy before resection may have some benefit in receiving the same combination of chemotherapy after resection. After failure of doxorubicin, dacarbazine (DTIC), and ifosfamide, there are no additional drugs for the treatment of soft tissue sarcomas that are of established value. The other somewhat active agents (methotrexate, etoposide, and interferon-alpha) have response rates of about 10%.
Other characteristics may suggest effectiveness or lack of effectiveness for various chemotherapeutic agents. Dhaini and colleagues46 evaluated human P450 isoenzymes, specifically CYP3A4/5, which aid in the metabolism and detoxification of carcinogens and chemotherapy. The authors found that patients with distant metastases were more likely to have elevated expression of CYP3A4/5 within the primary tumor biopsy specimen than patients without metastatic disease (p = 0.0004). The authors concluded that high levels of this human cytochrome P450 isoenzyme may be a marker to predict metastases or limited survival in patients with primary osteogenic sarcoma. Cyclooxygenase II (COX-II) enzyme levels did not correlate with primary or metastatic disease and the survival, as noted by Dickens and colleagues.47
A recommended practice is to consider patients with soft tissue sarcoma with one or two isolated lung lesions and a long disease-free survival for immediate surgery. For patients with more than two lesions, chemotherapy (Adriamycin, ifosfamide) could be used to assess a biological response. When maximal response has been achieved, resection can be performed and followed by additional chemotherapy. For unresectable metastases, chemotherapy may provide a response sufficient to allow surgical resection, after which additional chemotherapy may be considered. If chemotherapy is unsuccessful, surgery may be considered for palliation of symptoms. In marginal patients in whom chemotherapy provided only a minimal response or no change, surgery may be considered for local control of the metastases. Occasionally, metastases may grow to enormous size, compressing the heart and mediastinum (a “tumor thorax” or “tumor tamponade”) with the same consequences of tension pneumothorax or hemothorax. Chemotherapy is not commonly effective in this situation, given the need for urgent mechanical intervention. A heroic attempt at resection may be required. Cardiopulmonary bypass for cardiac decompression
and cardiopulmonary support may be required simply to manipulate the tumor within the thorax or mediastinum.
and cardiopulmonary support may be required simply to manipulate the tumor within the thorax or mediastinum.
Radiation Therapy
Currently, radiation therapy is used to palliate symptoms of advanced metastases (e.g., extensive pleural involvement, bone metastases). Radiation therapy is rarely used to treat pulmonary metastases. Prophylactic lung irradiation has been carried out in patients with osteogenic sarcoma. Burgers and colleagues30 reported that patients having prophylactic lung irradiation had similar rates of recurrence of pulmonary metastases as patients having postoperative adjuvant chemotherapy. More recently, Feigenberg and colleagues57 proposed whole-lung radiation therapy for patients with pulmonary metastases from giant-cell tumors of bone. Whelan and coworkers270 have suggested that lung radiation could be considered in selected patients with osteogenic or Ewing’s sarcoma for treatment of subclinical lung disease. The role of prophylactic lung irradiation in addition to current standard chemotherapy remains to be determined. Spunt and associates237 retrospectively reviewed the role of whole-lung irradiation in patients with pulmonary metastases from Ewing’s sarcoma who did not respond completely to induction chemotherapy. Only eight patients received irradiation. The 5-year survival rate was 37% in the treated population. There are no randomized studies to suggest that the addition of whole-lung radiation therapy improves outcome in patients with Ewing’s sarcoma.
Surgery
In selected patients with resectable pulmonary metastases and absence of extrathoracic metastases, complete resection is generally associated with improved long-term survival regardless of histology. In even more highly selected patients with extrathoracic metastases controlled or resected, resection of isolated pulmonary metastases may be considered to remove all known disease. An example of such a patient is one with colorectal carcinoma who has previously had hepatic metastases resected and is now discovered to have pulmonary metastases. In these patients, the thoracic surgeon may take advantage of the tumor biology, which limits metastases to the liver and lung. These patients have enhanced long-term survival compared with those with unresectable metastases.
Selection of Patients for Resection
Patients with isolated pulmonary metastases may be selected for resection. Clinical criteria have been proposed to identify and select patients who can benefit optimally from resection of their pulmonary metastases by McCormack,160 Mountain,170 and Pastorino183 and their coworkers as well as by Putnam and Roth197 (Table 126-1). Unfortunately, most patients with metastases do not benefit from surgery because of one or more of the following reasons: (a) a biologically aggressive tumor characterized by extensive disease, (b) a short disease-free interval (DFI) between control of their primary tumor and identification of pulmonary metastases, and (c) rapid metastatic growth.
Table 126-1 Excision of Pulmonary Metastases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
In patients being considered for resection, physical examination, radiographic examination, and physiologic assessment are performed to estimate the extent of resection and determine whether the planned procedure may be safely performed. Cardiac and pulmonary assessments are emphasized. In patients with preoperative chemotherapy or in those in whom pulmonary compromise is expected, a spectrum of pulmonary function tests are performed. These tests include spirometry with and without bronchodilators, diffusion capacity for carbon monoxide (DLCO), and oxygen consumption testing ([V with dot above]o2max). Echocardiography and an exercise stress test also may be needed.
In the operating room, the chest radiographs and chest CT scans are displayed prominently. After bronchoscopy, a double-lumen endotracheal tube is placed and used for anesthetic gas delivery. When a median sternotomy incision is used, sequential deflation of each lung aids in exposure and palpation of the pulmonary nodules. All nodules are resected with a margin of normal tissue. Nodules should not be “shelled out” because viable tumor cells remain on the periphery of the resected area. The margin should be adequate. Even when the margin is negative, microscopic cells may remain. Higashiyama and colleagues95 prospectively evaluated 51 patients with pulmonary metastases with an intraoperative lavage cytology technique for the surgical margin. They found that 11% of patients had a positive cytology at the margin despite having a rim of normal tissue. Additional tissue was then resected. Localized micrometastases may be present in some patients despite a macroscopically negative margin. This may contribute to subsequent local recurrence.
In general, the decision as to the adequacy of margin is the surgeon’s alone. After resection, the lung parenchyma may become distorted around the nodule, thereby giving the illusion of a positive or close margin to the pathologist. Mediastinal lymph node metastases rarely occur from pulmonary metastases, as Udelsman and coworkers252 have shown. However, in 2004, Ercan and colleagues56 reported on their experience with 70 patients who had had a complete mediastinal lymphadenectomy during pulmonary metastatectomy. These investigators found that 28.6% of their patients had involvement in the lymph nodes and that such involvement was a significant prognostic factor for poor outcome (3-year survival without nodal involvement, 69%, versus 38% with nodal involvement).
Table 126-2 Advantages and Disadvantages of Various Surgical Resections | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Is there a limit to the number of metastases that can be resected with associated survival benefit? Several authors have been tempted to address this specific question, including Putnam,199 Girard,77 Pastorino,183 and Robert208 and their colleagues. In general, only unresectability as defined by the thoracic surgeon should be considered as an absolute contraindication to resection. As the numbers of metastases increase, the potential for occult micrometastatic disease also increases. Although the surgeon may be able to extirpate all identifiable disease mechanically by visual inspection and palpation, the surgeon typically cannot identify or extirpate microscopic disease. The biology of patients with excessive numbers of metastases (but yet still “resectable”) is not changed by resection. Balancing the advantages of mechanical resection with the need for control of micrometastatic disease may be best accomplished in a multidisciplinary center with a multidisciplinary conference of all potential treating physicians. Pneumonectomy may be a consideration simply for mechanical palliation of mediastinal compression from “tumor thorax,” as shown by one of the present authors200 and Grunenwald88 and their associates. Pneumonectomy is rarely indicated to remove multiple metastatic nodules from one side of the chest.
Surgical Techniques and Incisions
Surgical procedures for resection include single thoracotomy, staged bilateral thoracotomies, median sternotomy, bilateral thoracotomy (the clamshell incision), minimally invasive techniques in selected patients, or other local control techniques. Patients with bilateral metastases may be safely explored with either a median sternotomy or staged bilateral thoracotomies, as noted by Johnston,111 and Roth and associates.213 The incisions chosen do not influence patient survival if all metastases are resected. Various advantages and disadvantages are unique to each approach (Table 126-2).
Patients with sarcomas and unilateral nodules often have multiple and bilateral metastases discovered during the operation. Bilateral metastases may occur in 38% to 60% of patients with preoperative unilateral sarcomatous metastasis. Postresection survival rates after median sternotomy or bilateral staged thoracotomies and complete resection are similar. A median sternotomy is performed for the initial exploration and resection in patients with bilateral nodules and may be considered as an initial procedure in patients with pulmonary metastases from osteogenic or soft tissue sarcomas or suspected bilateral metastases from any primary neoplasm in which wedge resections may be required. An exploration for unilateral or bilateral nodules as well as resection of these nodules may be accomplished through a median sternotomy incision.
Despite the previous discussion, for some unilateral disease, bilateral exploration may not be necessary. High-resolution CT may assist in this determination. Younes and colleagues279 evaluated the role of ipsilateral thoracotomy in patients with unilateral pulmonary metastasis for contralateral disease-free survival and overall survival. They noted that there was no significant difference in survival in patients who had recurrence in the contralateral lung compared with those who had bilateral metastases on admission. They suggested that delaying the contralateral thoracotomy did not affect survival.
Laser-Assisted Resection
Laser-assisted pulmonary resection, described by Kodama,123 Branscheid,28 Miyamoto,169 Landreneau,133 Mineo,166 and Rolle210 and their associates, using the neodymium:yttrium-aluminum garnet (Nd:YAG) laser, may provide a better means of
resecting pulmonary metastases than the surgical stapler. Use of the laser may enhance preservation of lung parenchyma with less distortion. Bovie electrocautery may also spare lung parenchyma by removing the metastases with minimal distortion of remaining lung (cautery excision). Air leaks, if they occur, can be sealed by oversewing the parenchymal defect or by the use of fibrin glue. Disadvantages of laser resection may include longer operating time and a potential for prolonged postoperative air leaks. Newer laser technologies have been developed. Rolle and colleagues210 describe a new 1,318-nm Nd:YAG laser for better and more precise incisions into the parenchyma with concurrent coagulation and sealing of the lung tissue. A 5-mm rim of tissue destruction is achieved.
resecting pulmonary metastases than the surgical stapler. Use of the laser may enhance preservation of lung parenchyma with less distortion. Bovie electrocautery may also spare lung parenchyma by removing the metastases with minimal distortion of remaining lung (cautery excision). Air leaks, if they occur, can be sealed by oversewing the parenchymal defect or by the use of fibrin glue. Disadvantages of laser resection may include longer operating time and a potential for prolonged postoperative air leaks. Newer laser technologies have been developed. Rolle and colleagues210 describe a new 1,318-nm Nd:YAG laser for better and more precise incisions into the parenchyma with concurrent coagulation and sealing of the lung tissue. A 5-mm rim of tissue destruction is achieved.
In a prospective randomized trial conducted by Mineo and colleagues,166 use of the Nd:YAG laser for resection of lung metastases was examined in 45 patients. The authors identified that the use of the laser reduced hospital stay, air leak, and tissue loss; however, a survival advantage was not proved. The use of the laser for resection of pulmonary metastases is oncologically equal to other techniques and may provide advantages by preserving lung tissue and minimizing associated surgical trauma.
Median Sternotomy and Clamshell Incision
For the median sternotomy incision, the patient is positioned supine with the entire anterior thorax exposed from the neck to the umbilicus and laterally to each anterior axillary line. The sternum is divided. The pulmonary ligament is divided on each side to mobilize the lung completely. The lungs are sequentially deflated and palpated. Metastases are identified and resected, and then the deflated lung is reinflated. The deflated right lung may be brought completely into the field, attached by only the hilar structures. Exposure of the left lower lobe may be more difficult than exposure of the other lobes because of the overlying heart. With appropriate gentle traction on the pericardium, the left lower lobe can be exposed quite readily and brought into the operative field. Various techniques to better visualize the lung may be used, such as surgical packs behind the hilum of the deflated lung to elevate the parenchyma or an internal mammary artery retractor to expose basilar tumors or posterior hilar left lower lobe masses. In certain circumstances visualization can be supplemented by the use of video thoracoscopy. Relative contraindications to median sternotomy include obesity, chronic obstructive pulmonary disease, elevated hemidiaphragm (particularly on the left), and cardiomegaly. Patients with metastases involving the left hilum or the posterior or medial portions of the left lower lobe may benefit from bilateral staged thoracotomies rather than median sternotomy. A median sternotomy in these situations may compromise completeness of resection and injure lung parenchyma or create a need for greater pulmonary resection than would be otherwise required.
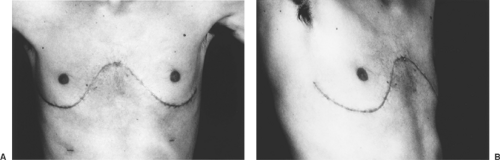
The clamshell incision, as described by Bains and coworkers,12 is a modification of the median sternotomy incision. Originally, this approach developed from the early days of carinal surgery and was later rediscovered for access to enhance bilateral sequential single-lung transplantation. A curvilinear incision is made under the breasts or pectoral muscles (Fig. 126-3A). The pectoral muscles are elevated to gain access to the fourth intercostal space bilaterally, whereupon the chest is entered and the incision carried to the sternum bilaterally. The most lateral aspect of the incision may curve superiorly toward the axilla (Fig. 126-3B). The sternum is divided transversely at the level of the fourth intercostal space with a Gigli or oscillating saw. After placement of a chest retractor for both the right and left thorax, the chest is opened, giving excellent exposure to right and left thorax, hilum, and mediastinum. Advantages of this approach include better exposure of the left hilum
posteriorly and the left lower lobe. Disadvantages include a large, painful incision and some difficulty with sternal reconstruction and stabilization.
posteriorly and the left lower lobe. Disadvantages include a large, painful incision and some difficulty with sternal reconstruction and stabilization.
Thoracotomy
The posterolateral thoracotomy is a familiar and standard approach to pulmonary resection for carcinoma of the lung. Posterolateral thoracotomy (with or without sparing of the latissimus dorsi muscle) may provide better exposure for metastases located more medially or more posteriorly near the hilum on the left side. In addition, for patients with bulky metastases, a posterolateral thoracotomy provides good access for faster resection and optimal sparing of lung parenchyma. The surgeon is typically limited to operating in one hemithorax. Bilateral thoracotomies are rarely performed in the same patient at the same operation, although left thoracotomy after median sternotomy may be performed safely in selected patients.
The vertical axillary thoracotomy may also be considered. Margaritora and colleagues153 have described their experience with staged axillary thoracotomy. Hospitalization was short (3.2 days). Operative trauma was minimal, as was postoperative pain. The interval between the two staged procedures was about 24 days.
Bilateral anterior thoracotomy as described by d’Amato and coworkers43 may also be used. Bilateral minithoracotomy with video assistance was used as an alternative to the other surgical approaches to the chest.
Video-Assisted Thoracic Surgery
Video-assisted thoracoscopic resection using high-resolution video imaging may be helpful for the diagnosis, staging, and resection of metastases. Its usefulness is limited, however, because metastases can be identified generally only on the surface of the lung or the outer 10% to 20%, depending on size. Metastases within the lung parenchyma may be undetectable with this technique. In one early report, Landreneau and associates134 have described minimal morbidity and no mortality in 61 patients who underwent 85 thoracoscopic pulmonary resections. Lesions were small (<3 cm) and in the outer one third of the lung parenchyma. Metastases in 18 patients were resected through thoracoscopy in this series. Video-assisted thoracic surgery (VATS) was the only procedure performed in these patients. Thoracoscopy may readily be used for diagnosis of metastatic disease, as stated by McCormack and coworkers,162 but its use in the treatment of metastatic disease is more controversial. In an elegant study, McCormack and colleagues163 prospectively evaluated VATS resection for the treatment of pulmonary metastases. Patients were screened with CT, and VATS was performed on all patients. Under the same anesthetic, thoracotomy or median sternotomy was performed. The authors found more nodules at thoracotomy than had been noted at the VATS procedure. Limitations of the study were the inclusion of patients with multiple metastases or prior sarcoma histology and screening with older CT scans. VATS is not the standard approach for resection in patients with pulmonary metastases. However, VATS may be considered in highly selected patients with a solitary nodule and nonsarcomatous histology on high-resolution (spiral) chest CT. Patients with sarcomatous histology frequently (40%–60%) have occult metastases, which may be palpated and resected with open thoracotomy.
More recently, Landreneau and colleagues135 recorded their experience in 80 patients with colorectal metastasis who underwent thoracoscopic resection of pulmonary metastasis. A single lesion was removed in 60 patients, and two or more lesions were removed in 20 patients. The overall 5-year survival rate was 30.8%. The authors required that all lesions identified on CT be identified at thoracoscopy or the minimally invasive approach would be abandoned. If location of the lesion compromised a complete resection, conversion to thoracotomy was performed. Accurate, high-resolution CT is critical for the selection of patients for minimally invasive techniques, as reported by Nakajima and colleagues.173 Lin and colleagues147 also noted that the results appeared comparable to historical results by open thoracotomy. The need for high-resolution helical CT scanning was crucial for patient selection. In addition, conversion to an open procedure was recommended when preoperative lesions were not identified or when surgical margins would be compromised.
To balance the need for palpation of the lung parenchyma in addition to minimizing trauma with thoracoscopy, Mineo and colleagues167 retrospectively evaluated transxiphoid palpation of both lungs during VATS for lung metastasis. Bilateral palpation was performed in 65 of 74 patients. Twenty-three radiographically undetected malignant lesions were identified. The authors recommended that this technique be considered as a blended approach to minimize thoracic trauma while providing for palpation of the lung parenchyma.
At present, VATS can be advocated for diagnosis or staging of the extent of metastases, or for resection of metastases in highly selected patients (i.e., those with solitary, nonsarcomatous histology in peripheral location on high-resolution spiral CT).
In patients with solitary metastasis from solid tumor adenocarcinoma or squamous cell carcinoma, careful consideration must be given to excluding primary lung carcinoma, which would require lobectomy and systematic mediastinal lymph node dissection for optimal care. Complications of VATS may include not resecting all metastases, positive margins, or pleural seeding with extraction of the metastasis (Fig. 126-4), as shown by Walsh and Nesbitt263 as well as by Ang6 and Downey50 and their coworkers. Follow-up on all patients is necessary at regular intervals because the likelihood of recurrence remains for a period of time.
Results of Resection of Pulmonary Metastases
The results of resection for pulmonary metastasectomy require critical analysis of factors that may potentially influence survival. Analysis of results should be based on review or study of single primary histology (breast, colon, melanoma) or similar histology (e.g., soft tissue sarcomas) and sufficient numbers of patients. Prognostic indicators have been reviewed to assess their influence singularly and in combination on postresection survival in patients with pulmonary metastases and to assist clinically in describing appropriate patients for resection of pulmonary metastases. Age, gender, histology, grade, location of the primary tumor, stage of primary tumor, disease-free interval between resection of the primary tumor and the appearance of the
metastasis, number of nodules on preoperative radiologic studies, unilateral or bilateral metastases, tumor doubling time (TDT), and synchronous or metachronous metastases may be evaluated preoperatively. Postoperatively, extent of resection, technique of resection, nodal spread, number of metastases and location, re-resection, postthoracotomy disease-free survival, and overall survival may also be considered in selecting patients for resection of pulmonary metastasis.
metastasis, number of nodules on preoperative radiologic studies, unilateral or bilateral metastases, tumor doubling time (TDT), and synchronous or metachronous metastases may be evaluated preoperatively. Postoperatively, extent of resection, technique of resection, nodal spread, number of metastases and location, re-resection, postthoracotomy disease-free survival, and overall survival may also be considered in selecting patients for resection of pulmonary metastasis.
Pastorino and associates183 reviewed the long-term results of resection of pulmonary metastasis based on an International Registry of Lung Metastases. This International Registry was established in 1991 based on 5,206 patients with pulmonary metastases and treatment collected from Europe, the United States, and Canada. Various clinical characteristics were compared in a retrospective yet consistent and controlled manner. Eighty-eight percent of these patients had complete resection. A solitary metastasis was resected in 2,383 patients; multiple lesions were resected in 2,726. Epithelial histology predominated (2,260 patients), followed by sarcoma (2,173), germ cell (363), and melanoma (328). With a median follow-up of 46 months, actuarial survival was 36% at 5 years, 26% at 10 years, and 22% at 15 years. For incomplete resection, actuarial survival was 15% at 5 years. The multivariate analysis showed several favorable prognostic indicators: resectable metastases, germ cell tumors, disease-free intervals (DFIs) of ≥36 months, and a solitary metastasis. In this international and multi-institutional study, the overall operative mortality rate was 1%; the mortality rate was 2.4% after incomplete resections and 0.8% after complete resections.
The most frequently performed operation was unilateral thoracotomy (58% of patients). Bilateral exploration was performed through either bilateral synchronous or staged thoracotomy (11%) or median sternotomy (27%). Thoracoscopy was performed in only 2% of patients. Wedge resections (67%), segmentectomy (9%), lobectomy or bilobectomy (21%), and pneumonectomy (4%) were also performed. Only 26% of patients had ≥4 metastases. Only 9% had ≥10 metastases, and 3% had ≥20. Multiple metastases were most commonly resected in sarcomas (64%), germ cell tumors (57%),
epithelial tumors (43%), and melanoma (39%). Metastases to the mediastinal lymph nodes were uncommon. Overall, 3% had redo surgery, 15% had two operations, 4% had three operations, and 1% had four or more operations. The maximum number of resections performed on a single patient was seven.
epithelial tumors (43%), and melanoma (39%). Metastases to the mediastinal lymph nodes were uncommon. Overall, 3% had redo surgery, 15% had two operations, 4% had three operations, and 1% had four or more operations. The maximum number of resections performed on a single patient was seven.
The authors proposed a system by which patients can be grouped into prognostic categories. These would include three parameters: (a) resectability, (b) DFI, and (c) number of metastases. In patients with resectable lesions, a DFI <36 months and multiple metastases were found to be independent risk factors. In resectable patients, therefore, three clinically distinct groups could be identified: (a) no risk factors, DFI of ≥36 months, single metastasis; (b) one risk factor, DFI <36 months, or multiple metastases; and (c) two risk factors, DFI <36 months, and multiple metastases. Group d consisted of all the unresectable patients. The authors noted that median survival was 61 months for group a, 34 months for group b, 24 months for group c, and 14 months for group d. The discriminant power of the model was appropriate for epithelial tumors, soft tissue sarcomas, and melanomas.
The value of this International Registry of Lung Metastases lies in its large collection of patient characteristics. These clinically identifiable characteristics may be reexamined and analyzed for various hypotheses. The limitations of such a registry lie in not accounting for variables in the biological behavior of these metastases. This variable behavior may be explained by molecular characteristics on which the clinical characteristics are based. This clinical database has been used to evaluate the value of resection of pulmonary metastases from various histologies as well as other clinical and molecular characteristics that may be valuable in selecting patients for optimal care of their metastases.
Extended Resection of Pulmonary Metastases
Of all patients undergoing resection of pulmonary metastases, <3% require an extended resection. Pneumonectomy or other extended resection of pulmonary metastases may be performed safely in selected patients with associated long-term disease-free survival. Pneumonectomy or en bloc resection of pulmonary metastases with chest wall or other thoracic structures, such as diaphragm, pericardium, or superior vena cava, have been performed in a small number of patients with good results, as noted by Putnam and associates.200 Nineteen patients had a pneumonectomy, and 19 patients had other extended resection. The 5-year actuarial survival rate was 25%. The mortality rate was 5%, and these deaths occurred in patients having pneumonectomy, often after multiple prior wedge resections for metastases.
In a French study by Spaggiari and colleagues,236 42 patients were treated for pulmonary metastases over 10 years: 29 patients underwent pneumonectomy for sarcoma, 12 for carcinoma, and 1 for a lipoma. Most tumors were centrally located. Two postoperative deaths occurred. Four patients had major complications. Five patients (12%) had recurrences in the contralateral lung. The median survival time was only 6.25 months, and the 5-year survival rate was 16%. Given that the standard surgical mortality rate for operations for pulmonary metastases is <1%, mortality for pneumonectomy should be considered in planning operations for patients with large, centrally located metastases. Although mortality for pneumonectomy for pulmonary metastases corresponds to mortality for other histologies, the 5-year survival rate of only 16% should prompt strict preoperative selection criteria. The authors suggest that young patients, those with a long DFI, and those with normal carcinoembryonic antigen (CEA) levels (for patients with metastases from colorectal carcinoma) be considered for pneumonectomy for pulmonary metastases.
Koong and coworkers124 also examined the value of pneumonectomy for metastases by retrospective review of the International Registry of Lung Metastases. Of the 5,206 patients enrolled, 133 (2.6%) had undergone pneumonectomy for pulmonary metastases between 1962 and 1994. Eighty-four percent of these patients underwent complete resection, and the 30-day mortality rate was 3.6%. The 5-year survival rate was 20% with complete resection. For incomplete resection, the perioperative mortality rate was 19%, and most did not survive beyond 5 years. The authors identified favorable prognostic factors of (a) single metastasis, (b) negative mediastinal lymph nodes, and (c) complete resection (R0). The authors concluded that pneumonectomy may be performed safely with adequate long-term survival.
These studies reveal that pneumonectomy is rarely indicated for pulmonary metastases. All the above series represent a highly selected group of patients and although the results are encouraging, pneumonectomy should be used only in rare circumstances as the surgical treatment of metastatic disease. Most if not all prognostic variables should be favorable before recommending pneumonectomy.
Larger or rapidly growing metastases may compress the mediastinum, or a uniquely positioned metastasis may impinge on or invade cardiac structures or great vessels. The use of cardiopulmonary bypass or other cardiovascular surgical techniques may allow resection of these metastases with palliation of symptoms and the potential for cure. Vaporciyan and colleagues258 reviewed a single-institution experience of resection with cardiopulmonary bypass of metastatic noncardiac primary malignancies. Patients with inferior vena cava tumors were excluded. Nine patients with sarcomas required cardiopulmonary bypass because their tumors directly involved the heart and the great vessels. The mortality rate was 11%. Of the 11 patients who underwent resection with curative intent, 10 had a complete resection. The use of cardiopulmonary bypass may be considered in highly selected patients, particularly when complete resection is anticipated.
Intra-atrial extension of sarcoma through the pulmonary vein is rare but may also be safely treated with pulmonary resection (pneumonectomy and resection of the tumor from the left atrium). Careful palpation of the involved vein can be performed intraoperatively, but excessive manipulation can result in tumor embolization. Extracorporeal cardiopulmonary support is required for a complete, safe, resection, as noted by Heslin94 and Shuman228 and their associates.
Osteogenic Sarcoma
Goorin and associates82 and Huth and Eilber103 reported that pulmonary metastases from osteogenic sarcoma may occur in up to 80% of patients who relapse after treatment for their primary
neoplasm, whether or not they receive adjuvant chemotherapy. CT is commonly used to identify patients with potential metastases. The positive predictive value for chest CT may be limited. Often, the surgeon finds twice the number of nodules than otherwise would be expected simply on the basis of preoperative CT of the chest, as described by Picci and colleagues.190 Resection as initial therapy for solitary metastasis, resection as salvage after chemotherapy for these pulmonary metastases, and multiple repeat thoracotomies may all be considered in selecting an optimal therapeutic strategy.
neoplasm, whether or not they receive adjuvant chemotherapy. CT is commonly used to identify patients with potential metastases. The positive predictive value for chest CT may be limited. Often, the surgeon finds twice the number of nodules than otherwise would be expected simply on the basis of preoperative CT of the chest, as described by Picci and colleagues.190 Resection as initial therapy for solitary metastasis, resection as salvage after chemotherapy for these pulmonary metastases, and multiple repeat thoracotomies may all be considered in selecting an optimal therapeutic strategy.
Meyer and associates165 reported that because metastases from osteogenic sarcoma are often isolated to the lungs, resection may render a significant number of patients disease-free and enhance long-term survival. The 5-year survival rate may range up to 40%, as shown by Snyder235 and Belli20 and their coworkers. Patients may have benefit regardless of the time of identification of lung metastases. In one Japanese study, Tsuchiya and colleagues250 noted that a longer DFI was associated with improved 5-year survival. Still, 2-year survival from the time of identification of pulmonary metastases ranged from 24% to 33% for patients with lung metastases identified at initial presentation, during preoperative chemotherapy, or during postoperative chemotherapy. Patients with lung metastases that occurred or were identified after completion of chemotherapy had a 2-year overall survival of 40% and a 5-year survival rate of 31%. However, in a small study, Yonemoto and colleagues278 evaluated 117 patients with osteogenic sarcoma of the extremity; 9 patients had pulmonary metastases at presentation. Patients who were treated with chemotherapy and aggressive resection had a 5-year survival rate of 64%.
Carter,34 Jaffe,108 and Putnam198 and their associates have evaluated survival and prognostic factors in patients with pulmonary metastases from osteogenic sarcoma. In a series from the National Institutes of Health, Putnam and colleagues198 evaluated 80 patients with osteogenic sarcoma of the extremity. Of these, 43 patients developed pulmonary metastases and 39 underwent one or more thoracic explorations for resection of their metastases. The 5-year survival rate was 40%. Various prognostic factors were analyzed. Three or fewer nodules, longer DFI, resectable metastases, and the fewer metastases identified and resected were associated with longer postthoracotomy survival. Resection was not possible if >16 nodules were identified on preoperative tomograms. A multivariate analysis did not find any combination of factors to be more predictive than the number of nodules identified on preoperative tomograms. In a more recent study by Heij and coworkers90 of 40 children with osteogenic sarcoma, it was found that incomplete excision, lack of primary tumor control, and progression and development of metastases during treatment were all negative prognostic factors. Surprisingly, in resectable patients, the number of metastases, DFI, unilateral versus bilateral metastases, preoperative and postoperative adjuvant treatment, and the number of thoracotomies performed were not significant prognostic factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree