Chapter 19 Secondary mitral regurgitation (MR) is the most common valve disease. A population-based study combined the echocardiographic data from three separate studies funded by the National Institutes of Health (NIH) that examined young (Coronary Artery Revascularisation in Diabetes [CARDIA]), middle-aged (Atherosclerosis Risk in Communities [ARIC]) and older adults (Cardiovascular Health Study [CHS]) to determine the incidence of moderate to severe valvular disease. Their findings in approximately 12,000 adults demonstrated that valve disease was equally common among men and women and among blacks and whites, and that it increased in frequency with age. MR was the most common significant valve disease, with an incidence less than 1% before age 55 years but increasing each decade and reaching more than 9% after age 75 years. 1 The incidence of MR was similar among the residents of Olmsted County. 1 Patients with MR had larger ventricles without hypertrophy. This analysis did not differentiate between primary and secondary MR. However, a later meta-analysis attempted to examine the prevalence of MR in the U.S. population and categorize the type of MR according to Carpentier’s classification (see Chapter 21). Although there were a number of limitations to the methodology, the analysis estimated that MR affected 2 to 2.5 million people in the United States in the year 2000. The largest group could be classified as having Carpentier type IIIb, with restricted motion due to left ventricular (LV) dysfunction, either ischemic or nonischemic. 2 The investigators of the meta-analysis estimated the prevalence of MR due to ischemic cardiomyopathy at 7500 to 9000 per million, and of MR due to LV dysfunction at 16,250 per million. The high number of individuals affected by secondary MR warrants an in-depth understanding of its pathophysiology, diagnosis, and management. The mitral apparatus consists of the leaflets, annulus, chordae tendineae, papillary muscles, and supporting LV myocardium. 3 In contrast to organic mitral valve disease, most secondary MR is best thought of as a ventricular, rather than valvular, process. Mitral valve competency relies on a balance between the forces that close the valve and tethering forces that prevent the valve from prolapsing into the left atrium ( Figure 19-1). The papillary muscles normally help counterbalance the force of LV contraction on the mitral valve by exerting force parallel to LV contraction and perpendicular to the mitral leaflets, thus preventing mitral valve prolapse. In secondary MR, altered geometry and reduced contractility, either global or regional, result in mitral valve incompetence. FIGURE 19-1 Principles of mitral valve tethering in ischemic mitral regurgitation. Reduced contractility with dilation of the inferior wall in isolation or in association with global LV dilation results in lateral displacement of the papillary muscle and a longer distance from the papillary muscle tips to the mitral annulus. Consequently, altered tension on the chordae tendineae results in incomplete mitral leaflet closure. The term “papillary muscle dysfunction” may be misleading because it implies that an isolated reduction in the contractility of the papillary muscle is responsible for the MR. Rather than suffering from reduced contractility, the papillary muscle becomes tethered as a result of changes in the supporting LV wall and lateral displacement of the papillary muscle. 4 This process was elegantly shown by Kaul et al, 5 who demonstrated that hypoperfusion of the papillary muscle without an effect on other LV segments did not result in MR. However, global ischemia resulting in LV dilation and dysfunction with normal papillary muscle perfusion did cause incomplete mitral valve closure and MR. 5 Thus the papillary muscle is better thought of as a functional unit, composed of the papillary muscle and the subtending LV wall foundation. Alterations in LV geometry, chiefly increased sphericity, change the position of the papillary muscle and the direction of tension exerted on the mitral leaflets. The normal papillary muscle position allows them to exert vertical tension on the chordae and leaflets, preventing prolapse. However, when the papillary muscles are laterally displaced due to LV dilation, the direction of force on the mitral leaflets is altered and inhibits proper closure. The leaflets become tethered and the zone of coaptation reduced. This is seen on echocardiography as tenting of the mitral valve. In the VALsartan In Acute myocardial iNfarcTion (VALIANT) study, tenting area, coaptation depth, annular dilation, and left atrial (LA) size were associated with the extent of baseline MR, but the degree of tenting after MI was the only variable that independently and significantly predicted progression of MR. A tenting area greater than 4 cm2 was associated with the highest risk of MR progression after MI. 6 In the setting of acute inferior MI, typically caused by right coronary artery or left circumflex CAD, a resultant inferior wall motion abnormality leads to tethering of the posteromedial papillary muscle and a loss of support for the medial aspects of the anterior and posterior mitral leaflets. On echocardiography the posterior leaflet appears to have restricted motion toward the annulus, and the anterior leaflet appears to override the posterior leaflet without rising above the annular plane. This appearance has been termed pseudoprolapse. 7 The altered leaflet coaptation leads to a posteriorly directed jet and may result in silent MR. The extent of infarction may not necessarily correlate with the degree of MR. For the reasons described previously, patients with inferior MI may be more vulnerable to MR than those with anterior MI. Although anterior MI may affect a larger area of myocardium, the LV remodeling from inferior MI may involve a smaller area of myocardium with less global LV dilation but may cause more dramatic alterations in the papillary muscle geometry ( Figure 19-2). FIGURE 19-2 Ischemic mitral regurgitation in inferior versus anterior myocardial infarction. Occasionally, active ischemia may cause “flash” pulmonary edema for which MR may be a contributing factor. However, ischemic MR need not imply the presence of active ischemia. 8 It usually reflects the consequences of chronic CAD, essentially postinfarction MR remodeling. In fact, many patients with ischemic MR are not found to have reversible ischemia, and conversely, persistent moderate or severe MR can occur in 77% of patients who have already undergone revascularization with coronary artery bypass grafting surgery (CABG), in whom presumably the ischemic substrate was addressed. 9 Papillary muscle rupture is a different entity from other causes of ischemic MR. Infarction of the papillary muscle can occur in the setting of a relatively small MI, usually with infarction of the posteromedial papillary muscle because it receives blood from a single artery. The magnitude of MR depends on the site of papillary muscle rupture. Rupture of the head of the papillary muscle results in MR of a similar degree to that encountered with chordal rupture. Rupture of the body of the papillary muscle results in acute loss of support of half of the anterior leaflet and half of the posterior leaflet; the resultant MR is torrential and often immediately fatal. 10 MR severity is usually assessed by physical examination or echocardiography in the resting state. However, in the setting of coronary heart disease, exercise, labile hypertension, or other stressors may provoke dynamic changes in LV wall motion or otherwise result in further alterations of LV function and geometry. Dynamic changes in LV size and function may result in exercise-induced worsening of MR, thus contributing to heart failure symptoms. Addressing this question, Pierard and Lancellotti 8 looked at two groups of patients with LV dysfunction: one with and one without a history of acute pulmonary edema. The two groups were matched for resting MR severity, LV size, and EF. Despite similar heart rates and blood pressure responses to exercise, those patients with a history of acute pulmonary edema were more likely to have significant exercise-induced increases in MR volume, regurgitant orifice area (ROA), and pulmonary pressure. 8 The unmasking of significant MR with exercise may explain the clinical conundrum of patients who experience dyspnea on exertion that seems out of proportion to their resting LV function, resting MR grade, or degree of stress-induced ischemia. Conversely, evaluation of MR during intraoperative transesophageal echocardiography (TEE) may underestimate true MR severity because sedation and inotropic agents can temporarily reduce LV size, improve LV function, and reduce papillary muscle tethering, thus complicating surgical decisions about procedures to reduce MR at the time of CABG. Thus, MR is best evaluated under normal loading conditions in the preoperative setting. 11 In patients with dilated cardiomyopathy, the mitral annulus is dilated and the papillary muscles are abnormally splayed. The papillary muscle architecture results from spherical remodeling of the ventricle. The chordae are stretched and the leaflets become tethered such that the point of coaptation lies within the LV chamber rather than in its normal position closer to the mitral annulus. There is a smaller total area of coaptation, which leads to valvular incompetence. In addition, the reduced closing force on the mitral leaflets due to diminished contractility contributes to the smaller area of coaptation. Thus, the extent of mitral closure depends on the balance between the tethering forces due to chordal stretch and the closing forces during systole. 12 In 1972, on the basis of the work of early investigators,13–15 Roberts and Perloff 10 formulated a postulate for the mechanism of MR in patients with dilated cardiomyopathy. The papillary muscles are located in the middle to apical third of the ventricle. In the normal elliptically shaped left ventricle, the contraction of the papillary muscles exerts a vertical force on the leaflets, which brings them together during isovolumic contraction and prevents them from prolapsing into the left atrium during ejection. As the ventricle becomes more spherical owing to remodeling, the papillary muscles migrate laterally and can no longer exert vertical force during systole, leading to reduced apposition of the leaflets with subsequent incompetence. As MR leads to further LV dilation, there was early recognition that “MR begets MR.” In an experimental model of dilated cardiomyopathy created through sequential coronary microsphere embolization, progressive MR was observed. 16 The first parameter to change prior to the onset of MR was the sphericity of the left ventricle measured at end-systole. Increases in LV volume and mitral annular diameter ensued and were associated with worsening of the MR. The coaptation depth, measured as distance from the mitral annulus to the tips of the leaflets, was increased at the onset of MR and did not increase further over time. In additional experiments, the investigators observed that MR occurred only in dogs with increases in sphericity. 17 In clinical studies, the primary dependence of MR severity on LV sphericity is less certain. In a study of 128 patients with LV dysfunction (LVEF <50%), including patients with both ischemic and nonischemic etiologies, there was a correlation of ROA and sphericity. However, in multivariable analysis that included measures of mitral valve deformation, this index was no longer significant. 18 A later study confined to patients with nonischemic cardiomyopathy also showed that sphericity correlated with MR but was not an independent predictor of severity. 19 Further observations on the depth of coaptation in functional MR were extended to include more robust measures based on two-dimensional (2D) and three-dimensional (3D) echocardiography, including tenting area and tenting volume, respectively. Yiu et al 18 examined patients with both ischemic and dilated cardiomyopathy and found that tenting area measured in the parasternal long-axis view on 2D echocardiography was related to posterior and apical displacement of the papillary muscles and correlated with ROA. The tenting area (cm2) values were 6.66 ± 0.9, 7.46 ± 0.9, and 8.86 ± 1.5 in patients with ROA (mm2) values of less than 10, 10 through 19, and more than 20. 18 In another study by Karaca et al, 19 tenting area was the best parameter to predict severe functional MR (ROA >20 mm2) at a cutoff level of 3.4 cm2 with 82% sensitivity and 77% specificity. 19 In a sheep model of pacing-induced dilated cardiomyopathy, the 3D tenting volume correlated best with the MR severity and was predicted by the severity of annular dilation rather than subvalvular remodeling. 20 A clinical study of 37 patients with functional MR used 3D echocardiography to measure maximal and minimal tenting volumes during systole as well as tenting areas on 2D apical long-axis, two-chamber, and four-chamber views. The best predictor of ROA was the tenting volume, measured at end-systole (minimum). These researchers defined the optimal cut-point for minimal tenting volume as 3.90 mL or larger, which identified significant functional MR (ROA >20 mm2) with a sensitivity of 86% and a specificity of 100%. 21 Annular dilation and shape also contribute to functional MR in patients with dilated cardiomyopathy. As the annulus dilates, the amount of leaflet tissue required to effectively occlude the annulus during systole increases occurring at the expense of coaptation zone area; in other words, the valve is closed but not sealed. Annular dilation exceeding a critical value eventually results in noncoaptation and MR. Using 2D echocardiography, Boltwood et al 22 demonstrated that mitral annular area was significantly larger in patients with dilated cardiomyopathy with MR than in those without MR and that the total leaflet area, derived mathematically, was significantly greater. LA size and mitral annular area were the major determinants of leaflet area and mitral regurgitant severity, whereas LV size was less important. 22 These findings suggest that the leaflet area relative to annular area in systole determines the extent of MR. This concept was supported by a study using 3D echocardiography in 44 patients with MR related to bilateral papillary muscle displacement. The investigators showed that the area of leaflet coaptation was significantly lower in patients with hemodynamically significant functional MR than in those without. They defined coaptation area as the difference between the leaflet area at the onset of systole and that in mid-systole. Coaptation length was measured at three sites: medial, middle, and lateral. The annular and the leaflet areas were greater in those with significant functional MR (cm/m2) (annular: 6.8 ± 1.6 vs. 5.4 ± 0.9; leaflet: 9.2 ± 1.9 vs. 8.3 ± 1.6). The ratio of leaflet area to annular area and the coaptation length were also lower in the presence of significant MR. 23 In addition to the specific anatomic properties in dilated cardiomyopathy, mechanical factors are important, specifically the force and coordination of LV contraction. The closing force on the leaflets, or transmitral pressure, is related to LV systolic pressure, which varies during systole. Schwammenthal et al 24 examined the instantaneous regurgitant orifice using M-mode echocardiography to measure the proximal flow convergence divided by instantaneous velocity. They demonstrated that in dilated cardiomyopathy there was a decrease in ROA throughout systole with an increase during LV relaxation, compared with a relatively constant ROA in rheumatic MR and an increase in ROA during systole in mitral valve prolapse. 24 Using an in vitro model, He et al 12 demonstrated the contributions of papillary muscle position, apical displacement of the papillary muscles, annular dilation, and the driving pressure. 12 Apical and posteromedial displacement of the papillary muscles increased both leaflet tethering and MR, as did annular dilation, whereas higher driving pressures reduced ROA and decreased MR. The severity of MR in their model varied during systole as in the clinical observation described earlier. Delayed closure of the mitral valve due to increased tethering caused early systolic MR. In mid-systole, the closing forces were maximal, and the regurgitant orifice size was at a minimum. As LV pressure fell, there was an increase in MR in late systole. An additional clinical study demonstrated that mitral annular area decreased during systole, but this change had a smaller contribution to the decrease in regurgitant orifice than the progressive rise in transmitral pressure. 25 Intraventricular dyssynchrony due to conduction defects has become an important therapeutic target of the use of biventricular pacing for cardiac resynchronization therapy (CRT). CRT has been shown to reduce MR in clinical trials. 26 LV dyssynchrony may contribute to MR through a number of different mechanisms, including uncoordinated contraction of the papillary muscles with alteration in the timing of tethering forces exerted on the leaflets and reduced closing forces on the leaflets. However, the role of dyssynchrony is likely overridden by factors related to mitral deformation, as demonstrated in a clinical study using tissue Doppler imaging to derive the standard deviation of the time to peak systolic contraction as a measure of dyssynchrony. In this study, dyssynchrony contributed only weakly to MR severity after correction for tenting area and LV sphericity, and only in patients with nonischemic cardiomyopathy. 27 While dyssynchrony may only contribute a small part to the development of MR, improvement in synchrony with CRT may indeed relate to improved MR severity. For example, one study showed that reduction in MR with CRT was related to improved timing of coordinated contraction of the papillary muscles. 28 Another demonstrated that the improvements in total MR were related to reductions in LV end-systolic volumes and mitral valve tenting area. However, the reduction in early systolic MR was related to end-systolic volume and global dyssynchrony, whereas the reduction in late systolic MR was related to tenting area and dyssynchrony. 29 In the obstructive form of HCM, late systolic MR is associated with the systolic anterior motion of the anterior mitral leaflet (SAM) and is coincident with the onset of LV outflow tract obstruction. 10 In 1969, Wigle et al 30 performed a clinical study that demonstrated that MR was reduced when the outflow tract obstruction was eliminated or reduced through the administration of angiotensin or through surgery as long as there were no primary abnormalities of the mitral valve. Conversely, MR worsened with pharmacologic interventions that increased the severity of obstruction, such as isoproterenol and amyl nitrite. Jiang et al 31 further elucidated the mechanism of MR in HCM, demonstrating the following geometric contributions: anterior and inward displacement of the papillary muscles, anterior displacement of the anterior leaflet, and elongation of the anterior mitral leaflet. The displacement of the papillary muscles was believed to reduce the support of the central portions of the leaflets, causing them to slacken and to be subject to greater anterior drag. In a pathologic study of 43 mitral valve specimens from patients with HCM and basal outflow tract obstruction, 19 had enlarged, elongated mitral valves. The echocardiograms of these patients showed that their valves were situated more posteriorly and that they had greater systolic excursion of the anterior leaflet, which showed a more sharp-angled bend and localized contact of the tip with the septum. The echocardiograms of the patients with normal-sized leaflets showed more anteriorly situated valves with septal contact involving a greater portion of the valve. Those with normal-sized anterior leaflets were more likely to have diffuse thickening of the anterior leaflet that restricted the SAM, preventing the sharp right-angled bend. 32 The geometric relationships of the papillary muscles to the LV outflow tract may be important in predicting outcomes after septal ablation. Delling et al 33 measured echocardiographic dimensions that reflected the malposition of the anterior leaflet (anterior-to-posterior leaflet coaptation position ratio) and the distance between the papillary muscle and anterior septum relative to the left ventricular internal diameter as well as the anterior position of coaptation relative to the septum (coaptation-to-septum distance). The patients who demonstrated persistent SAM after alcohol septal ablation had more severe anterior malposition at baseline and were more likely to have persistent obstruction. There are numerous reported cases of ruptured chordae tendineae contributing to MR in patients with HCM. A surgical case series demonstrated that in the majority of patients with MR due to chordal rupture, the posterior leaflet was affected. At surgery, the leaflet tissue appeared normal, unlike in patients with degenerative mitral valve disease. The investigators in this series hypothesized that the cause was related to increased stress on the posterior leaflet, which is perpendicular to flow during systole. 34 The mechanisms for MR in these patients are as diverse as the underlying etiologies. In amyloid heart disease, there may be primary valvular involvement due to amyloid deposition. However, functional MR may be associated with severe LA enlargement and annular dilation. In Fabry disease, mild MR is frequent but it is rarely hemodynamically significant. 35 In Loeffler endocarditis associated with hypereosinophilic syndrome, MR due to scarring and fibrosis of the chordae tendineae is common, occurring in almost 50% of patients. MR contributes to congestive heart failure, and valve surgery may be required.36,37 However, because even bioprosthetic valve replacement may be complicated by thrombosis in this setting, long-term anticoagulation should be considered. 36 It is well known that patients with hemodynamically significant primary MR are at risk for development of atrial fibrillation and that the occurrence of atrial fibrillation is associated with poor prognosis. 38 However, it has only been recently recognized that atrial fibrillation can lead to MR in patients with anatomically normal mitral valves. 39 In an observational cohort study of patients undergoing ablation for atrial fibrillation, the investigators studied 53 patients with normal mitral valves and normal LV function who had moderate to severe MR and compared them with a matched cohort of patients with grade mild or less MR. These patients represented approximately 7% of the cohort referred for ablation in whom preprocedural echocardiograms had been obtained. Patients with moderate or severe MR were older, more likely to be in persistent atrial fibrillation, and had a higher incidence of hypertension. They had greater LA volumes and larger annular dimensions. Mitral annular dimension was the strongest predictor of significant MR with an odds ratio of 8.4 per cm of annular size. The strongest evidence that atrial fibrillation caused MR was provided at follow-up. Only 18% of patients who maintained sinus rhythm still had moderate to severe MR, compared with 82% of those with recurrence of atrial fibrillation. Only the patients who maintained sinus rhythm had a significant reduction in annular dimension, suggesting that the primary cause of atrial functional MR is annular dilation. This concept is not universally accepted. An earlier study compared patients with atrial fibrillation alone and patients with ischemic or dilated cardiomyopathy. Despite similar annular dimensions and annular areas in the two groups, the degree of MR, as measured by regurgitant fraction (RF), in those with atrial fibrillation was very modest (RF = 3%) compared to those with cardiomyopathy (RF = 36%). The investigators concluded that papillary muscle tethering due to LV dilation was the major cause of MR and that annular dilation did not have an important role. 40 One important difference in the studies is the method of measuring MR. In the study of patients undergoing ablation, MR severity was determined by the ratio of jet area to LA area, which is less rigorous that the measurements of RF used in the other study. Although the observation that atrial fibrillation with secondary atrial enlargement and annular dilation can lead to MR is probably valid, it occurs in less than 10% of patients and the degree of MR is usually not severe. Hemodynamically significant tricuspid regurgitation is more common, probably because the fibrous skeleton of the tricuspid annulus is less developed than that of the mitral valve. 41 MR may be suspected from history and physical examination. Dyspnea is the predominant symptom associated with MR. However, in most patients with secondary MR, symptoms related to the underlying condition predominate; dyspnea and fatigue may result from ischemia or from nonischemic cardiomyopathy rather than the MR per se. In addition, the typical holosystolic murmur of MR may be absent. In acute MR associated with ischemia or infarction, the murmur is often early systolic and may be high pitched or “cooing” in quality. In a small Thrombolysis In Myocardial Infarction (TIMI) substudy in the post-MI setting, a murmur was appreciated in only 50% of cases in which MR was clearly present on contrast-enhanced left ventriculography. 42 Even with moderate to severe MR, only two thirds of patients had appreciable murmurs.43,44 When the MR is directed posteriorly, as occurs with an inferior wall motion abnormality and tethering of the posterior leaflet, the murmur may radiate to the back and may be missed on routine precordial examination.
Secondary Mitral Regurgitation
Epidemiology
Pathophysiology
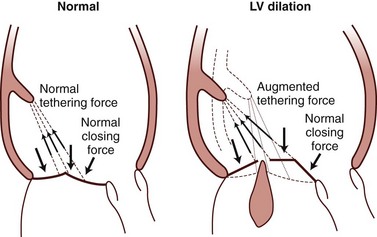
Basic principles of tethering mechanism for ischemic mitral regurgitation (MR) and balance of apposed closing and tethering forces acting on the leaflets. Augmented tethering force created by papillary muscle displacement apically displaces the leaflets and causes MR. LV, Left ventricular.
Secondary Mitral Regurgitation in the Presence of Coronary Artery Disease
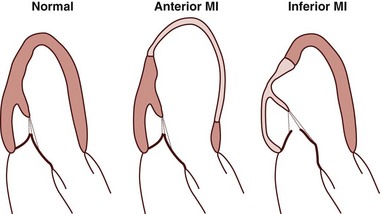
Potential mechanism for higher incidence of ischemic mitral regurgitation in inferior versus anterior myocardial infarction (MI), despite the lower level of global left ventricular (LV) remodeling in patients with inferior MI. LV remodeling in anterior MI may involve a broader region of the LV without causing major alterations in the mitral valve complex. In contrast, LV remodeling in response to inferior MI may involve less area but may cause major alterations in the mitral valve complex.
Dynamic Changes
Functional Mitral Regurgitation in the Absence of Coronary Artery Disease
Dilated Cardiomyopathy
Hypertrophic Cardiomyopathy
Restrictive Cardiomyopathy
Atrial Functional Mitral Regurgitation
Diagnosis
General
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree










