CHAPTER 23 Secondary Lung Tumors
After the liver, the lung is the second most common site for metastatic involvement in neoplastic disease when all tissues and organs are considered, and 20% to 54% of patients with cancer will have pulmonary metastases at some point in the natural history of their disease (Boxes 23-1 and 23-2). In the absence of extrathoracic metastases (i.e., in about 25% of patients with disseminated disease), complete resection is associated with increased survival, regardless of histology. With appropriate patient selection, life expectancy is often improved with pulmonary metastasectomy. Cures are reported with either resection alone or in combination with chemotherapy.1,2 Even in the context of unresectability, surgical forms of palliation may serve to improve quality of life. For other patients (e.g., those with nonseminomatous germ cell tumors), surgery may have a more diagnostic role, such as defining residual disease that is potentially amenable to salvage forms of therapy.
HISTORY OF METASTASECTOMY
During the 19th century, there were sporadic reports of lung resections for metastatic tumors in the European literature. The first of these reports was in 1855, by the French surgeon Sédillot, who removed a chest wall tumor and excised disease extending into the lung.3 The first case of a true pulmonary metastasectomy was described by Weinlechner in 1882 in a patient with a rib sarcoma, who was found to have two incidental pulmonary metastases at the time of the sarcoma resection.4 Kronlein reported the first long-term survivor after pulmonary metastasectomy in a patient with recurrent chest wall sarcoma and a metastatic lung nodule. The patient went on to survive 7 years, eventually succumbing to recurrent pulmonary disease.5–7
The first, and perhaps most famous, report of a planned pulmonary metastasectomy in the United States was performed in 1933 by Barney and Churchill.1 Soon after resection of a renal cell carcinoma, they noted that the patient’s pulmonary nodule, seen on a chest radiograph preoperatively and presumed to be tuberculosis, had doubled in size. The lesion, now thought to represent metastatic disease, was treated with radiation therapy. They noted a poor response and elected to resect the nodule. The patient went on to live 23 years, eventually dying of coronary artery disease with no evidence of tumor recurrence at autopsy. Despite the early discovery that survival could be improved by resection of metastatic disease, it was not for another 40 years that metastasectomy was performed as a separate procedure by Divis in Europe.8 This was followed soon after by similar reports in the American literature by Torek and Tudor Edwards in the early 20th century.9,10
These early reports, and others like them, paved the way toward general acceptance of pulmonary metastasectomy. Although initial indications for surgery were reserved for those with a solitary metastasis, with time and experience more aggressive metastasectomies were performed. In 1947, Alexander and Haight described the first case series of 24 patients who underwent pulmonary metastasectomy.11 In this series, they described a woman, in her 20s, with a spindle cell neurogenic sarcoma. She initially underwent a right lower lobectomy for metastatic disease in 1939. She had a recurrence in 1940 for which she underwent a left upper lobectomy. This article was the first to define criteria for resection of pulmonary metastases, including control of the primary tumor, absence of extrathoracic disease, and sufficient pulmonary reserve. Today, the indications for resection of secondary pulmonary malignancies have been broadened to include not only patients with recurrent disease but also those with multiple metastases, bilateral lesions, and essentially all histologies.12
The largest effort aimed at evaluating patients undergoing pulmonary metastasectomy was undertaken by the International Registry of Lung Metastases (IRLM). Established in 1991, the IRLM accrued 5206 patients in North America and Europe. This landmark report demonstrated that complete resection, short disease-free interval, and single lesions were favorable measurements for long-term survival. This and other studies have helped to develop current criteria for performing pulmonary metastasectomies and to define anticipated survival after resection.13,14,66
PATHOPHYSIOLOGY OF PULMONARY METASTASES
In 1889, the British surgeon Stephen Paget observed that metastatic disease followed a nonrandom pattern. Using autopsy records of patients with a variety of primary tumors, he hypothesized that factors in certain tumors have an affinity for certain factors in target organs. Theories have subsequently evolved that attempt to explain the propensity for metastases to spread to specific organs. The “cascade spread” theory hypothesizes that a single organ represents the first site of spread, followed by systemic dissemination.15 This metastatic cascade is considered to be a complex series of events culminating in the generation of metastases. The initial phase of the cascade involves tumor growth via neovascularization, which is stimulated by growth factors secreted by the tumor cells and local host cells. The invasive phase of tumor growth involves the local production and activation of proteolytic enzymes (matrix metalloproteinases, collagenases, serine proteinases, cysteine proteinases) derived from both the host and tumor tissue. These enzymes serve to decrease cell adhesiveness, stimulate cell migration, and enhance chemotaxis and subsequent tumor cell detachment.
Movement from the extracellular space into a vascular compartment is termed intravasation. Cancer cells have been shown to degrade basement membranes via local release of extracellular matrix-degrading proteins (matrix metalloproteinases), facilitating migration into the lymphatic and circulatory system. Once in the circulatory system, tumor cells must avoid recognition and destruction by the host immune system. Only 0.1% of tumors cells in circulation go on to generate metastases.16 Mechanisms that cancer cells use to survive include human leukocyte antigen (HLA) class I downregulation (mediator of immune cell recognition) and loss of immunogenic antigens. Cancer cells also downregulate the immune system via the production of immunosuppressive cytokines. Some evidence suggests that clots form around tumor cells that protect these cells from immunologic and physiologic stresses in the bloodstream.17
Tumor cells bind to pulmonary vasculature which, via a platelet-induced reaction, stimulates endothelial cell retraction.18 The lung is thought to play a role as the primary capillary filter for drainage of most organs, and its rich capillary network provides an ideal environment for deposition. Movement of tumor cells from the circulatory system into the interstitium is called extravasation. Basement membrane disruption is thought to occur in a manner similar to intravasation.
Once extravasated, tumors may remain quiescent or proliferate. Proliferation and local invasion requires neovascularization, which is induced via a shift toward the intracellular and extracellular production of pro-angiogenic factors. Epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor α (TGF-α) foster tumor cell proliferation in the new environment. On the other hand, the host organ produces inhibitors, such as TGF-β, mammastatin, and amphiregulin, to prevent metastatic implantations.19 These compounds are under investigation to assess their ability to control metastatic disease.
Do Metastases Metastasize?
It is thought that metastases need to follow the same steps as the primary tumor to metastasize: angiogenesis, intravasation, arrest, and extravasation. In 1975, Hoover and Ketcham demonstrated, experimentally, that metastases do have the ability to metastasize.20 In their experiment, the primary tumor in mice was amputated after pulmonary metastasis developed. These mice were then placed into parabiosis with normal syngeneic partners. Metastases were demonstrated in the non–tumor-bearing partners, supporting the theory that metastases can re-metastasize. In addition, both autopsy and experimental data have defined the concept of metastases from metastatic disease.21 On the other hand, Sugarbaker and colleagues took pieces of healthy lungs and transplanted them into mice that had established pulmonary metastases. Their results demonstrated no evidence of secondary tumor development, this leading to the conclusion that metastases do not metastasize.22
Whether metastases metastasize is still poorly understood and remains a challenge for medical oncologists and surgeons alike as they struggle to determine the best treatment and the proper timing of that treatment for patients with pulmonary metastases.23
Does Lung Cancer Metastasize to Lung?
Lung cancer can metastasize via lymphatic channels to the ipsilateral lung and, as suggested by some autopsy series, less commonly to the contralateral lung. These are patients with one primary lung cancer and intrapulmonary metastases. According to the proposed seventh revision of the lung cancer staging system of the International Association for the Study of Lung Cancer (IASLC), ipsilateral nodules in the same lobe as the primary tumor will be considered T4 and ipsilateral nodules in a different lobe from the primary tumor will be considered M1.24 It is difficult, however, to determine if these patients have synchronous lesions or a primary lung cancer with intrapulmonary metastases. Ichinose and coworkers have used DNA flow cytometry to evaluate these lesions.25,26 Using this technique, lesions are determined to be synchronous if they demonstrate completely different DNA ploidy. If both tumors show diploidy, or when at least one DNA index of abnormal clones between two aneuploidy tumors is the same or almost identical, they are considered metastatic. In addition, loss of heterozygosity and p53 mutational status have been used to distinguish multicentric lung cancers from intrapulmonary metastases.27,28 Using these criteria, it appears that lung cancer can metastasize to lung, albeit less commonly than synchronous tumors. These molecular genetic techniques will continue to be used to determine whether two lung lesions are synchronous primary lung tumors or a single primary lung tumor with metastases.
EVALUATION OF THE PATIENT WITH SECONDARY PULMONARY TUMORS
Symptoms and Presentation
Approximately 75% to 90% of patients with secondary pulmonary malignancies are asymptomatic and, therefore, their disease is most commonly discovered incidentally on routine or follow-up radiologic examinations.29,30 The usual lack of symptoms may, in part, be secondary to the common, nonobstructing peripheral location of pulmonary metastases. The asymptomatic nature of pulmonary metastases emphasizes the importance of obtaining lung imaging studies in the follow-up of cancer patients.
Symptoms, when they do occur, typically result from a delayed diagnosis with endobronchial or pleural involvement, large bulky disease, or central tumors. Patients may present with cough and hemoptysis suggesting an endobronchial lesion and thus warranting bronchoscopic examination. Endobronchial metastatic lesions are extremely rare, less than 2%, in patients who die of solid tumors, with breast, kidney, pancreas, colon, and melanoma as the most common sources.31 Another presenting symptom may be dyspnea, which is usually secondary to airway obstruction, a pleural effusion, parenchymal replacement by multiple metastatic lesions, or lymphatic spread. Finally, chest pain, wheezing, or pneumothorax may occur, but these are unusual presenting symptoms.
During physical examination, wheezing may be heard, which is a sign of airway obstruction. Occasionally, a pericardial rub is heard, representing pericardial involvement. Pleural and pericardial involvement are usually the result of ovarian, breast, or lung adenocarcinomas. Thymomas are notorious for their pleural involvement when they metastasize.32 In addition, decreased breath sounds and egophony may be appreciated when an obstructing lesion is present with associated lobar or segmental atelectasis, which could be seen on a chest radiograph as postobstructive pneumonia.
Radiographic Evaluation
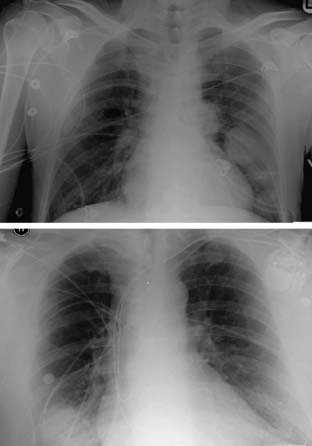
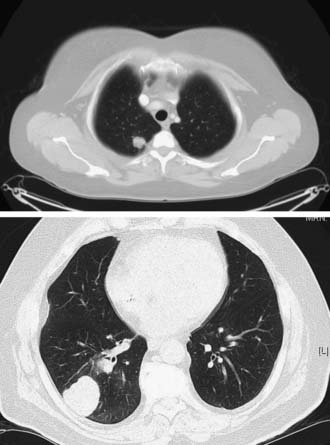
Most pulmonary metastases are detected by chest radiography (Fig. 23-1) or computed tomography (CT) (Fig. 23-2) during routine follow-up for the primary cancer. Radiologic testing is used to define the extent of the disease and to determine whether an individual is a candidate for pulmonary metastasectomy. A search for extrathoracic disease may involve imaging the original primary disease site as well as looking for distant disease. A new pulmonary nodule in a patient with a prior malignancy should be considered cancer until proven otherwise.
A standard chest radiograph is often obtained as a screening test in patients with prior malignancies, and it provides a baseline for future studies. The sensitivity of plain radiography is low in comparison to that of CT. The resolution of a routine chest radiograph is approximately 9 mm, whereas that of a CT scan is 1 to 2 mm. CT is the gold standard imaging modality for evaluating the lungs and characterizing metastatic lesions. Lung metastases tend to be small, usually less than 1 cm, and they appear as multiple lesions in 75% of cases. The majority, nearly 75%, of secondary malignancies appear with multiple lung nodules. Distinguishing between a metastatic lesion and a new lung primary carcinoma can be difficult. Most metastatic lesions to the lung are found on the lung periphery, and they have a predilection for the basilar lung fields, probably because of increased blood flow to this region when the patient is in an upright position.33,34
CT characteristics of metastatic lesions include spherical shape with well-circumscribed, smooth borders. Lesions with irregular or spiculated borders with associated linear densities are more commonly associated with primary lung cancers, although this is not a universal finding.33 Calcifications in lung nodules are rarely seen in metastatic pulmonary disease. Metastases that may have associated calcifications include osteosarcomas, chondrosarcomas, and breast and ovarian primaries. Cavitation can occur in both benign and malignant lesions. Benign etiologies include lung abscesses, aspergillosis, and tuberculosis. Malignant nodules that cavitate include squamous cell carcinomas (primary lung or metastatic carcinomas), sarcomas, and testicular tumors.
Although CT is useful for identifying and characterizing pulmonary metastases, it has been shown to underestimate the number of lesions compared with thoracotomy. In 1996, McCormack and colleagues prospectively performed video-assisted thoracoscopic surgery (VATS) resections based on CT findings, followed by thoracotomies with lung palpation, and found additional lesions in 14 of 18 patients.35 Parsons and coworkers confirmed that helical CT missed metastases in 47% of cases.36 Other studies have shown that, at least for patients who underwent metastasectomy for colorectal cancer, there is no statistically significant difference in survival between the open and the thoracoscopic approaches.37 Furthermore, as CT scanning technology evolves, the detection of nodules as small as 1 mm can be achieved, further narrowing the disparity between CT scanning and manual palpation.38 At this point, the data support thoracotomy and manual palpation as being the more sensitive method for detecting nodules. However, the choice between thoracotomy and VATS involves many factors and should be individualized for every patient.
Positron emission tomography (PET) has proved to be beneficial when considering patients for metastasectomy. In a series of patients with melanoma, [18F]fluorodeoxyglucose (FDG)-PET had a sensitivity of 92%, a specificity of 88%, and accuracy of 91%.39 PET is helpful in assessing extent of disease, including the primary disease site as well as thoracic and extrathoracic potential sites of metastases, but it is limited by false-negative results in patients with sub-centimeter pulmonary metastases.
The mediastinum should be evaluated carefully, because metastatic disease involves the mediastinum 2% to 3% of the time.31,40 Head and neck tumors, testicular tumors, renal cell carcinomas, breast carcinomas, and melanomas have been known to appear with mediastinal disease.
Tissue Diagnosis
Sputum cytology is generally not indicated, as it is often nondiagnostic because of the peripheral location of most pulmonary metastases.41 Bronchoscopic examination and diagnosis of pulmonary metastases is useful in the case of endobronchial or centrally located lesions. Fine-needle aspiration (FNA) is another option for obtaining a tissue diagnosis. It is associated with a risk of pneumothorax, in some series as high as 27%.42 In addition, the sensitivity of this procedure may be relatively low (80%). Equivocal or “negative” results do not establish a diagnosis of benign disease. Generally, this procedure is thought to yield useful information only when it establishes a diagnosis of cancer.43 When a nonsurgical therapy is indicated for a suspected histologic diagnosis, or when a patient is not a surgical candidate, FNA represents an excellent diagnostic option. Success of this procedure depends not only on the skill of the operator but also on the diagnostic prowess of the interpreting cytologist. The emerging field of bronchoscopic biopsies using electromagnetic navigation has demonstrated promising results and has potential to fall into the preferred armamentarium of clinicians attempting to establish a diagnosis for lung nodules.44 The role of this technology in the care of patients with pulmonary metastatic disease has not yet been established.
For excisional biopsy, VATS has a sensitivity and specificity approaching 100%.45–47 It is a more significant procedure than FNA and requires general anesthesia with selective lung ventilation. It is indicated when FNA has failed to or is unlikely to establish a diagnosis, or when more information or tissue is required for treatment purposes. Also, under appropriate circumstances, it may be therapeutic as well as diagnostic, as the nodules can be completely excised.
Occasionally, there is a role for thoracoscopic FNA, such as in cases of multiple lesions not amenable to complete surgical resection when treatment hinges on a tissue diagnosis and all less-invasive diagnostic modalities have proved fruitless or are deemed less safe than a VATS approach.48
INDICATIONS FOR SURGERY
Does the pulmonary nodule represent one site of multiorgan spread, thereby contraindicating resection? Pulmonary metastases are common in the advanced stages of cancer, with as many as one third of patients presenting with secondary nodules. Of these patients, however, the majority of pulmonary nodules (75% to 85%) are a manifestation of widespread disease. Consequently, only 15% to 25% of patients have lesions confined to the lung and are appropriate candidates for curative resection (Table 23-1). Preoperative evaluation, therefore, should exclude extrathoracic disease.
When pleural or pericardial effusions are present, thoracentesis or pericardiocentesis should be performed to rule out malignancy. Positive cytology contraindicates resection. Cytology may be falsely negative, however, in up to 40% to 60% of cases.49,50 Consequently, a negative cytology returned in the context of a high index of suspicion for malignancy warrants pericardial or pleural biopsy, or both. A VATS approach can be used to access the pleural space, and it offers a minimally invasive transthoracic approach to the pericardium. It also affords the option of visualizing and palpating the lung as well as biopsying the mediastinal lymph nodes. Bronchoscopy should always be performed before thoracoscopy or thoracotomy, to rule out endobronchial lesions.
Are the lesions resectable? Unresectability is defined as noncontiguous involvement beyond the visceral pleural envelope.29 This is best determined at operation. Preoperatively, imaging modalities can only provide an estimate of resectability. In addition, thoracoscopy can sometimes be useful to assess for disseminated disease or bulky tumors that involve major structures and preclude complete resection, or to access a pleural or pericardial effusion that cannot be reached percutaneously. If the pericardium or pleura is involved with neoplastic disease from direct extension of an underlying parenchymal lesion, but there is no associated effusion, they should be resected en bloc with the specimen. Direct metastases to the pleura or pericardium in a discontinuous manner (i.e., disseminated spread) and malignant pleural or pericardial effusions are generally contraindications for resection.
Is the primary tumor controlled? Efficacy of pulmonary metastasectomy depends on, among other factors, the ability to control the primary neoplasm. The primary neoplasm, therefore, should generally be addressed before resection of the pulmonary metastases. Therefore, thorough preoperative testing should be performed to rule out other possible metastatic sites or local recurrence of the primary tumor prior to pulmonary metastasectomy (Box 23-3).
Box 23–3 Selection Criteria∗ for Metastasectomy
SURGICAL APPROACH
Surgery for pulmonary metastases is based on the principle of performing a complete resection while preserving as much lung tissue as possible in case metastasectomies are required in the future.51 The specific location of the tumor in the lung plays a role in determining the resection volume necessary to remove all disease. Wedge resection of the lung to include a negative resection margin is the standard therapy. Deeply located lesions or central lesions may require an anatomic resection—segmentectomy, lobectomy, or, rarely, pneumonectomy.
The choice of incision should reflect the extent of disease, the goal of complete resection, and the patient’s ability to undergo the procedure. The surgical options for metastasectomy include minimally invasive VATS and the traditional open procedure. VATS procedures typically involve three-port access to the pleural space, ipsilateral lung collapse via a double-lumen tube with digital palpation of the lung, and nodule localization through the nearest trocar site. Central lesions may be difficult to palpate through the trocar incision, which may justify an open procedure for their removal. Lin and colleagues reported on 99 potentially curative resections of metastases using a VATS approach and demonstrated long-term survival comparable to historic results with an open approach.52 Landreneau and coworkers observed less pain and a shorter hospital stay after VATS resection of colorectal metastases to the lungs.53 Critics of the VATS approach say that the loss of bimanual tactile ability results in a higher rate of missed metastases.
Median sternotomy, preferred by some surgeons, provides access to both thoracic cavities, but it provides limited exposure to lesions in the left lower lobe and large posterior central lesions. Some surgeons may find that central lesions necessitating lobectomy are more easily excised via a thoracotomy. Patients with a history of radiation therapy, obesity, chronic obstructive pulmonary disease, diabetes, and steroid use may be at higher risk for sternal complications after median sternotomy. Morbidity and mortality rates for lung metastasectomy by conventional means range between 0% and 31.6% and between 0% and 7.6%, respectively.54
The metastasectomy itself usually consists of a wedge resection. This is facilitated by the tendency of metastatic disease to be found at the periphery of the lung, and it is easily performed with lung clamps and a pulmonary stapling device. Excision with electrocautery or laser may also be performed under select circumstances.55–58 Posterior areas of the lung may be better assessed by filling the hemithorax with saline to float the lung anteriorly. Alternatively, posterior packs may be used. Only rarely are metastases found in regional lymph nodes, so formal nodal dissection is generally not indicated.29 Although in primary lung cancer, lobectomy has been shown to give higher survival rates than wedge resections, no such difference has been found with metastasectomy.59 Resection with a 1- to 2-cm margin of uninvolved tissue has proved to be adequate in this setting. Lobectomy is indicated if a lesser procedure would result in an incomplete resection or is not technically possible. This is most commonly the case with large, centrally located lesions or lesions in such close proximity to bronchovascular structures that an anatomic resection is required to avoid leaving fatally compromised lung tissue behind. Chest wall resection of contiguous lesions not associated with other disease, or even pneumonectomy, has been shown to improve survival when used in the appropriate setting.3,60,61
When considering a unilateral procedure for the treatment of presumed (on the basis of preoperative imaging) unilateral pulmonary metastases, the question is whether this approach will miss undiagnosed contralateral disease and whether this would be detrimental to the patient. One study tried to answer this question by looking at sternotomy versus unilateral thoracotomy in patients with metastases that appeared unilateral in preoperative imaging studies.62 Unilateral thoracotomy was used, and the contralateral lung was not assessed. One might expect residual disease in the unevaluated thorax, resulting in a worse prognosis for this group. In fact, no difference in long-term survival was found. Furthermore, repeat resection of recurrences have been shown to improve survival, thus making the argument that any missed subclinical disease could be resected subsequently.62,63 These results have been corroborated by Younes and colleagues.64 In this study, patients undergoing unilateral thoracotomy for unilateral disease (n = 179) and patients undergoing bilateral thoracotomies for bilateral disease (n = 88) were investigated. The two groups of patients with confirmed bilateral metastases (synchronous or metachronous) were compared. Patients who experienced recurrence in the contralateral lung within 3, 6, or 12 months had overall 5-year survival rates of 24%, 30%, and 37%, respectively. When patients with recurrence in the contralateral lung were compared with patients with bilateral metastases on admission, there was no significant difference in overall survival. The only two predictors of contralateral recurrence were histology and the number of pathologically proven metastases. The authors concluded that bilateral exploration of unilateral lung metastases is not warranted in all cases.64
A newer technique involves placement of a substernal handport used in conjunction with thoracoscopy. After thoracoscopy is performed, an epigastric incision is made, the linea alba is divided, and the xyphoid is usually excised. The substernal space is then entered by blunt dissection, and both pleural spaces are accessed, allowing manual lung palpation.65 Detterbeck and colleagues reported that this technique allowed adequate resection in 67% of patients; conversion to an open procedure was necessary in 33% of patients for anatomic and technical reasons.