CHAPTER 15 Screening for Lung Cancer
Lung cancer is the most common cause of cancer mortality in the United States, resulting in more deaths than breast, prostate, and colon cancer combined.1 Despite the accepted efficacy of screening for cancers of the breast, prostate, and colon, screening for lung cancer is generally thought to be not beneficial and even potentially harmful. The current recommendations from the National Cancer Institute (NCI) and from the American Cancer Society (ACS) are that no attempt at screening for lung cancer should be performed for patients thought to be at risk for this disease.2–4 According to the ACS, “Any test for the early detection of lung cancer” is not recommended, and “People with signs or symptoms of lung cancer should consult their physicians.”2 Given the high rates of lung cancer mortality (especially when signs or symptoms become apparent), combined with the lack of an effective screening tool, screening for lung cancer is the subject of active investigation at both the clinical and laboratory levels.
HISTORY OF SCREENING FOR LUNG CANCER
Interest in screening high-risk patients for lung cancer was sparked when the association between cigarette smoking and lung cancer was first appreciated in the 1950s.5 Plain chest radiography (CXR) was the first modality used in a mass screening trial to detect lung cancer. The first mass screening project was conducted in London from 1960 to 1964, and although it was not a randomized trial, 55,034 men were assigned to undergo either CXR every 6 months for 3 years (the screened group), or a single CXR at the beginning of the study, followed by a repeat CXR at the end of the 3-year period (the “unscreened” group).6 At the end of the 3-year period, more lung cancer was detected in the screened group, as a result of the screening protocol, than in the “unscreened” group (132 versus 96 cases). In addition, resectability was enhanced in the screened group. Despite these findings, lung cancer–specific mortality did not differ between the two groups, with 62 patients dying of lung cancer in the screened group and 59 patients in the “unscreened” group.6
Interest in lung cancer screening was renewed in the 1970s, when the NCI funded three randomized trials focused on the use of both CXR and sputum cytology.7–9 At the time, refinements in the technology used for the cytologic assessment of expectorated sputum encouraged the designers of two of these trials (the Johns Hopkins Lung Project and the Memorial Sloan-Kettering Cancer Center [MSKCC] trial) to focus primarily on the effect of the addition of sputum cytology to interval CXRs (Table 15-1).8,9 In the MSKCC study, patients were randomized to either annual CXR alone or annual CXR plus sputum cytologic assessment every 4 months.8 Exactly the same number of cancers were detected in both groups. Patients who had sputum cytology in addition to CXR tended to have their tumors detected at an earlier stage than those undergoing CXR alone. However, there was no difference in resectability rates or in lung cancer–specific mortality. This screening protocol was also used in the Johns Hopkins Lung Project randomized trial, with similar results (see Table 15-1).8,9 No difference in the number of lung cancers or in lung cancer–specific mortality was detected between the two groups.9
Table 15–1 Study Trials∗ Evaluating the Role of Sputum Cytologic Examination for Lung Cancer Screening
| MSKCC | Johns Hopkins | |
|---|---|---|
| Years of accrual | 1974–1982 | 1973–1982 |
| Screened arm | ||
| Sample size | 4968 | 5226 |
| Protocol | Annual CXR; sputum cytology every 4 mo | Annual CXR; sputum cytology every 4 mo |
| Cancers (baseline) (n) | 30 | 39 |
| Cancers (repeat screen) (n) | 114 | 194 |
| Lung cancer mortality† | 2.7 | 3.4 |
| Unscreened arm | ||
| Sample size | 5072 | 5161 |
| Protocol | Annual CXR | Annual CXR |
| Cancers (baseline) (n) | 23 | 40 |
| Cancers (repeat screen) (n) | 121 | 202 |
| Lung cancer mortality† | 2.7 | 3.8 |
CXR, chest radiography.
∗ The two randomized controlled trials are the Memorial Sloan-Kettering Cancer Center (MSKCC) trial (Melamed MR, Flehinger BJ, Zaman MB, et al. Chest 86:44-53, 1984) and the Johns Hopkins Lung Project (Tockman M. Chest 89:325S-326, 1986).
The design of the Mayo Lung Project was different from that of the MSKCC and Johns Hopkins trials: it focused on the combined impact of CXR and sputum cytology in screening for lung cancer (Table 15-2).7,10,11 In the Mayo trial, considered by many to be the most definitive of the four randomized trials, patients were randomized to undergo CXR as well as sputum cytologic assessment every 4 months for 6 years (the screened group), or given the standard Mayo recommendation to undergo both of these examinations annually (the “unscreened” group) without active reminders being sent to the participants.7 Significantly, in this trial, more than 50% of patients in the “unscreened” group underwent CXR during the study period, whereas 25% of patients in the screened group were not compliant with the screening protocol. After a median follow-up period of 3 years, more lung cancers were detected in the screened group than in the “unscreened” group. Both resectability and the 5-year survival rate were higher for individuals diagnosed with lung cancer in the screened group than in those with detected lung cancer in the control arm. Nevertheless, for the overall study population, there was no difference in lung cancer–specific mortality between the two arms.7
Table 15–2 Trials∗ Evaluating the Role of CXR Combined with Sputum Cytologic Examination for Lung Cancer Screening
| Mayo | Czechoslovakia | |
|---|---|---|
| Years of accrual | 1971–1983 | 1976–1980 |
| Screened arm | ||
| Sample size | 4618 | 3172 |
| Protocol | CXR and sputum cytology every 4 mo for 6 yr | CXR and sputum cytology every 6 mo for 3 yr† |
| Cancers (baseline) (n) | Data not available | Data not available |
| Cancers (repeat screen) (n) | 206 | 39 |
| Lung cancer mortality‡ | 3.2 | 3.6 |
| Unscreened arm | ||
| Sample size | 4593 | 3174 |
| Protocol | Advised for annual CXR and sputum cytology | CXR and sputum cytology initially and after 3 yr† |
| Cancers (baseline) (n) | Data not available | Data not available |
| Cancers (repeat screen) (n) | 160 | 27 |
| Lung cancer mortality‡ | 3.0 | 2.6 |
∗ The two randomized controlled trials are the Mayo Lung Project (Fontana RS, Sanderson DR, Taylor WF, et al. Am Rev Respir Dis 130:561-565, 1984) and the Czech Study on Lung Cancer Screening (Kubik AK, Parkin DM, Zatloukal P. Cancer 89[11 Suppl]:2363-2368, 2000).
† Followed by annual CXR and sputum cytology for an additional 3 years.
In the late 1970s, a screening trial was conducted in Czechoslovakia that was similar to the Mayo Lung Project; it also focused on the combined effects of CXR and sputum cytologic examination for lung cancer screening (see Table 15-2).7,10,11 In this trial, patients in the screened group underwent CXR and evaluation of sputum cytology every 6 months for 3 years, whereas those in the “unscreened” group had an initial CXR and sputum cytologic examination, both of which were repeated at the end of the 3-year period.10,11 After the initial screening period, both groups underwent annual CXR and sputum assessment for an additional 3 years. Once again, more lung cancer was diagnosed in the screened group than in the “unscreened” group (39 versus 27 cases). Despite this, no difference was appreciated in lung cancer–specific mortality.10,11 The interpretation of these four randomized trials continues to be the subject of some controversy. Opponents of lung cancer screening state that although three of these four randomized trials demonstrated that screening may indeed lead to diagnosis of more early-stage lung cancer and improvement in case-specific survival, there was no demonstrable reduction in lung cancer–specific mortality for the whole study population.12,13
Overdiagnosis bias has been cited as the most likely reason for this seemingly paradoxical result.12 Overdiagnosis implies that a significant number of cancers detected by screening are indolent and would not lead to clinical disease in these patients. Indeed, if all screen-detected lung cancers were to grow, spread, and become clinically apparent, it would be expected that the lung cancer incidence rates would be unaffected by screening. If followed long enough, all tumors would become symptomatic and would be detected at equal rates in both screened and unscreened patients. In contrast, if screening led to the diagnosis of lung cancers that were clinically indolent (i.e., overdiagnosis), these tumors would be diagnosed in the screened population but not in the unscreened group, resulting in higher rates of detection but no reduction in mortality in the screened group (as seen in the Mayo Lung Project and the Czechoslovak trials).
Proponents of lung cancer screening criticize the randomized trials using several arguments. First, none of the four randomized trials incorporated a completely unscreened control group, making any positive effects of screening more difficult to detect. Second, two of the trials (the MSKCC and Johns Hopkins trials) evaluated only the effect of sputum cytologic evaluation, as both the experimental and control groups had annual CXRs.8,9 Standard cytologic examination of expectorated sputum is not currently thought to be a sensitive means of screening for lung cancer. Furthermore, the patients diagnosed with lung cancer in both arms of these two trials had earlier-stage disease and higher resectability and survival rates compared with historical controls of sporadically diagnosed lung cancer patients, implying that annual CXR may be beneficial for lung cancer screening. Third, there was a significant amount of cross-over in the Mayo Lung Project, where more than half of the “unscreened” patients had CXRs, and 25% of the screened patients were not compliant with the protocol.7 Fourth, the Mayo Lung Project was designed to detect a 50% reduction in mortality and was underpowered to detect more subtle differences in mortality (i.e., it had a 19% chance of detecting a 10% decrease in mortality).14
In addition to these criticisms, advocates of screening argue that the concept of lung cancer overdiagnosis is erroneous on the basis of the following indirect evidence.15,16 First, lung cancer is a virulent disease, with nearly 90% of patients diagnosed with lung cancer dying of the disease, making the overdiagnosis hypothesis contrary to the known biological behavior of this disease process.1 Second, although the undiagnosed autopsy prevalence of some cancers (e.g., prostate) may be very high, this has not been found to be the case for lung cancer.17 Finally, data from the surgical literature, both prospective and retrospective, suggest that suboptimal treatment or no treatment of early-stage lung cancer is associated with an inferior prognosis when compared with optimally treated patients.18 Taken together, this information calls the assumption of overdiagnosis into question.
CURRENT LUNG CANCER SCREENING EFFORTS
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
The Prostate, Lung, Colorectal and Ovarian (PLCO) trial is a complex, multicenter trial sponsored by the NCI, with a target accrual of 148,000 subjects.19 Men and women, ages 55 to 74, were randomized to undergo an annual CXR (for 3 years [smokers] or 2 years [nonsmokers]) or routine medical care (unscreened group). Participants will be followed for at least 13 years after randomization to assess health status and cause of death. Accrual to the main phase began in 1994 and was completed in 2001. The PLCO trial has an 89% power to detect a 10% reduction in lung cancer–specific mortality. The results have yet to be reported. Unfortunately, the technology of film-based CXR used in this study has been routinely replaced by digital CXR. Additionally, CXR has already been shown to be inferior to computed tomographic (CT) scans for the detection of nodules, which will certainly lessen the impact of the results of the study.
National Lung Screening Trial
The National Lung Screening Trial (NLST) is an NCI-sponsored randomized controlled trial, previously described by Goldberg.20 It compares CT to CXR screening in current and former smokers, with baseline studies and then two annual follow-up studies. This trial is powered to detect a 50% reduction in lung cancer mortality within approximately 2 years of follow-up, and a 20% reduction within 6 years of the initial screening. As in the NCI studies performed in the 1970s, the primary endpoint is lung cancer mortality. In addition to the previously discussed limitations of CXR screening, a major concern in regard to the ongoing NLST relates to the number of rounds of screening and the length of follow-up. As is typical in modern screening trials, there are balances between cost concerns in the trial and the various endpoints. Although additional screening rounds would add confidence to the results, they are the most costly aspect of these trials and are therefore limited. In the NLST, there are only two annual repeat rounds, with a median follow-up of about 4 years. It is clear, based on previous studies, that significantly longer follow-up will be necessary before a genuine reduction in mortality can be expected. It thus appears that all of these ongoing studies are subject to critical design issues that might influence interpretation of their results.
Low-Dose Computed Tomography
In the 1990s, increased resolution and data-acquisition speeds of modern CT scanners generated renewed interest in screening for lung cancer. Initial findings from Henschke and colleagues of the Early Lung Cancer Action Project (ELCAP) showed that in a high-risk population, CT was superior to CXR in detection of lung nodules. Notably, 2.7% of those enrolled had lung cancer, the great majority of which were stage I.21,22 In the initial ELCAP (I-ELCAP) patient population, 27 screen-diagnosed lung cancers were found at baseline screenings, of which 96% were resectable.21 A subsequent report by the I-ELCAP group addressed overall curability estimated through 10-year survival rates of patients found to have stage I lung cancer by CT screening.23 The authors reported an estimated 88% 10-year survival rate, markedly higher than survival rates predicted by the current staging system or among those presenting as a result of symptoms. They inferred that because CT screening leads to early detection of lung cancer and because those lung cancers found as a result of CT screening are curable, that CT screening leads to a reduction in lung cancer mortality.
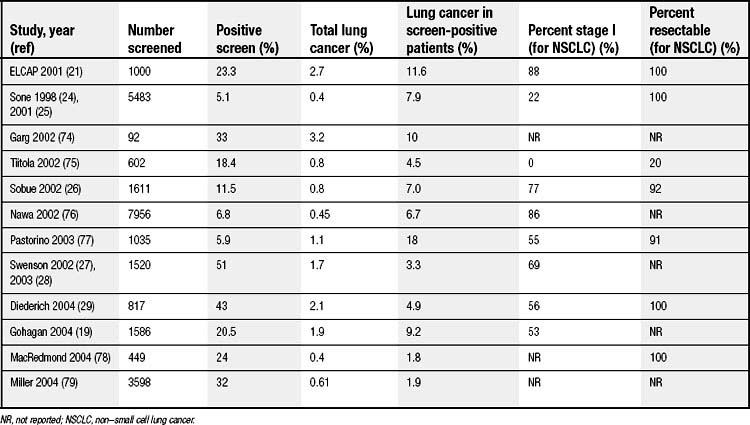
Several other groups have also evaluated CT screening for lung cancer. A recently published review by Black and colleagues identified 12 studies (Table 15-3),19,24–29 of which two were randomized and the other 10 had no comparator group.30 Significant variability existed in the study populations and in the definition of a positive finding in each. Nevertheless, the percentage of positive screenings ranged from 5.1% to 51%. From baseline screenings, 1.8% to 18% of positive findings led to a diagnosis of cancer. The majority of the tumors were in stage I (53% to 100%), with a high resectability rate (>78%). Only one of the studies reported 5-year survival: 76% for patients with cancer detected at baseline screening and 65% for patients with cancer detected at annual repeat scanning.31
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree