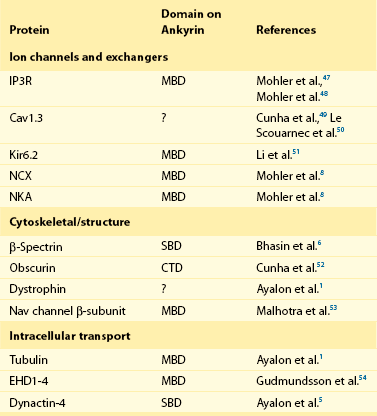
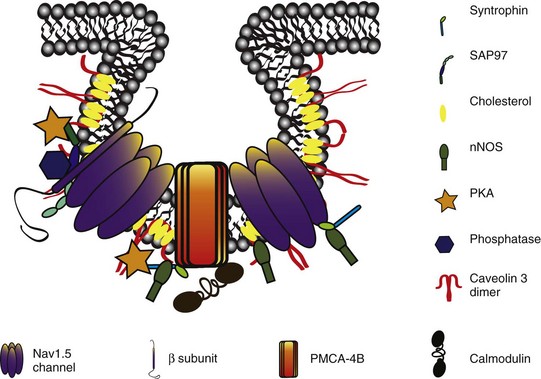
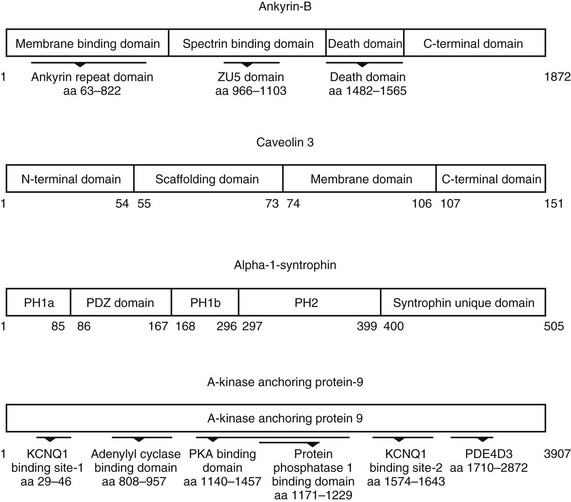
23 Scaffolding proteins are modular proteins that assemble multimolecular signaling complexes or macromolecular signaling complexes and that also modulate and regulate the function of associated proteins. The modular nature of their structure gives them the ability to associate with a multitude of signaling proteins via protein-protein interaction domains. In the heart, scaffolding proteins have also been shown to interact with ion channels and to modulate their function. Compelling evidence in the literature shows that scaffolding proteins associate with ion channels responsible for depolarization (sodium channels and both L-type and T-type calcium channels), ion channels responsible for repolarization potassium channels (time-independent, time-dependent, and voltage-gated delayed rectifiers), and gap junctions (connexin40 and connexin43) that connect cells electrically. The pore-forming protein of an ion channel is generally called the α-subunit, and other proteins thought to be associated with specific α-subunits are β-, δ-, or γ-subunits, or have other names such as minK. Although these subunits can assist in forming the complex and attaching the complex to the cytoskeleton and extracellular matrix and regulate channel surface expression or regulation, and although their associations may be less than specific in some instances, we have not included these subunits as a part of this review of scaffolding proteins that have more general interactions. The importance of scaffolding proteins and their role in the regulation of associated ion channels in physiology and pathophysiology has been highlighted by the discovery of mutations on scaffolding proteins that alter ion channel function and regulation. The focus of this chapter is specifically on the scaffolding proteins, mutants of which have been shown to induce long QT syndrome (LQTS). As an example, a schematic drawing of a macromolecular complex for the cardiac Na channel is depicted in Figure 23-1. Figure 23-1 A macromolecular complex representing channel-associated proteins, in this example the NaV1.5 sodium channel in a caveolar lipid membrane. The drawings are roughly representational of relative size, shape, and membrane associations. Protein-protein interactions are represented based on the literature, but no implication for where or how they are attached is intended. Ankyrins are a family of scaffolding proteins that link the membrane-bound proteins to the underlying cytoskeleton. This function of ankyrins contributes to the expression, specific localization, and overall stability of membrane proteins within the plasma membrane.1,2 The ankyrin family of proteins consists of three family members, ankyrin-R, ankyrin-B, and ankyrin-G encoded by genes ANK1, ANK2, and ANK3, respectively. Ankyrin-B and ankyrin-G have been detected in a variety of tissue. In contrast, ankyrin-R is detected only in erythrocytes, striated muscle, and some neurons.3 In general, an ankyrin protein has four functional domains, a membrane-binding domain (MBD), spectrin-binding domain (SBD), death domain, and a C-terminal regulatory domain (Figure 23-2). Figure 23-2 Schematic of scaffolding proteins (1) ankyrin-B, (2) caveolin-3, (3) a kinase anchoring protein-9 and (4) α1-syntrophin involved in LQTS 4, 9, 11, and 12, respectively. The N-terminal MBD contains 24 tandem ankyrin repeats; these ankyrin repeats have inherent spring-link qualities, and it has been suggested that this elastic property of the ankyrin repeat could have a role in mechanotransduction and confer pliancy to the MBD from mechanical perturbations.4 In addition, ankyrin repeats are also a common motif for protein-protein interactions found in many membrane proteins, such as sodium-calcium exchanger (NCX), sodium-potassium ATPase (NKA), ATP-sensitive potassium channels (Kir6), inositol 1,4,5 trisphosphate receptor (IP3R), and anion exchangers (Table 23-1). The SBD has been shown to bind to spectrin via its ZU5 domain (i.e., domains that are found in zona-occludens-1 [ZO-1] and the uncoordinated protein 5) that composes the minimal binding domain for spectrin. In addition to binding to spectrin, SBD associates with the regulatory subunit of protein phosphatase 2A (B56a), and dynactin4 an adaptor protein that links the dynein motor to membrane cargo.5,6 The death domain of ankyrin-G has been shown to interact with proteins involved in apoptosis,7 but the exact function of the death domain of ankyrin-B is currently unknown. The C-terminal regulatory domain, region of ankyrin proteins has the least homology, and this region confers different ankyrin family members their functional specificity and distinct subcellular localization. In cardiomyocytes, Mohler et al.8 identified an ankyrin-B based macromolecular complex comprising Na/K-ATPase (alpha 1 and alpha 2 isoforms), Na/Ca exchanger 1, and InsP3 receptor that were specifically localized in cardiomyocyte T-tubules in discrete microdomains distinct from classic dihydropyridine receptor/ryanodine receptor dyads. A mutation in ankyrin-B that caused LQTS was first described in 1995 by Schott et al.9 and was further defined in 2003 by Mohler et al.,10 who later in 2007 further identified ankyrin-B loss-of-function variants.11,12 These mutations caused a disruption in the localization of the sodium pump, the sodium–calcium exchanger, and inositol-1,4,5-trisphosphate receptors, which reduces the total protein level and targeting to T-tubules. In adult cardiac myocytes, ankyrin-B mutations altered Ca2+ signaling, caused extrasystoles, and provided a rationale for QT prolongation and associated arrhythmia. The variants of ankyrin-B were categorized into three distinct functional classes based on severity of clinical phenotype in associated patients and degree of ankyrin dysfunction in cardiomyocytes. The variants (G1406C, R1450W, and L1503V) associated with less severe clinical phenotype (or asymptomatic individuals) and a lesser degree of ankyrin dysfunction were put into class 1.11,12 The variants (T1404I, T1552N and V1777M) associated with more severe clinical phenotype and severe degree of dysfunction in cardiomyocyte were put into class 2. The variants (E1425G, V1516D, and R1788W) with the most severe clinical phenotypes and loss of function in cardiomyocyte were put into class 3.9–11 The phenotypes associated with these classes of variants included bradycardia, sinus arrhythmia, delayed conduction–conduction block, idiopathic ventricular fibrillation, and catecholaminergic polymorphic ventricular tachycardia. The severity of the loss of function of ankyrin variants has been directly linked to the inability of ankyrin to target NCX and NKA to the membrane.11 In a recent study, Camors et al.13, using an ankyrin-B heterozygous knockout (ankyrin-B+/–) mouse, demonstrated that myocytes from ankyrin-B+/– mice had approximately 20% reduced NCX and NKA protein expression with no significant change in the mRNA levels.13 They also reported that the calcium transients, SR calcium content, and fractional SR calcium release were significantly larger in the ankyrin-B+/– myocytes compared with wild type myocytes. Based on their experimental results, they concluded that the reason for the higher frequency of spontaneous diastolic calcium sparks in ankyrin-B+/– myocytes was caused by an alteration of the transport of sodium and calcium and enhancement of the coupled opening of ryanodine receptors. The results presented in the literature and discussed here suggest that additional work is necessary to elucidate the molecular mechanism underlying phenotypic variability of each human ankyrin-B variant. Caveolin is a protein that is essential to the formation of cavelike membrane structures called caveolae. Typically, caveolae are surface plasma-membrane invaginations or flask-shaped structures with a diameter of 50 to 100 nm. The caveolin family of proteins is encoded by three genes and consists of six known caveolin subtypes: caveolin-1α, caveolin-1β, caveolin-2α, caveolin-2β, caveolin-2γ, and caveolin-3. Of the six known caveolins, caveolin-3 is specifically expressed in muscle tissue, including the heart. Most of the information regarding the molecular structure of caveolins comes from the early studies on caveolin-1. Given that caveolin-1 and caveolin-3 are approximately 65% identical and 85% similar,14 it is necessary to discuss the structure of caveolin-1 and point out key differences between caveolin-1 and -3. The molecular structure of caveolin-3 is composed of 151 amino acids divided into four domains: the N-terminal domain (residues 1 to 54), the scaffolding domain (residues 55 to 73), the membrane domain (residues 74 to 106), and the C-terminal domain (residues 107 to 151; see Figure 23-2). The N-terminal domain of caveolin-1 is longer than that of caveolin-3 by 27 amino acid residues. The scaffolding domain, which is next to the N-terminal domain, is essential to the formation of caveolin oligomers and for associating with other proteins (Table 23-2). A W-W–like domain, a protein-protein interaction domain, overlaps the scaffolding domain and the membrane domain and is followed by a highly conserved proline residue.15 The W-W–like domain and the proline residues are conserved between caveolin-1 and -3, but absent in caveolin-2.15
Scaffolding Proteins and Ion Channel Diseases
Scaffolding Proteins Involved in Long QT Syndrome
Ankyrin-B (LQT4)
Structure and Associations
Mechanism of Action
Caveolin 3 (LQT9)
Structure and Associations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Scaffolding Proteins and Ion Channel Diseases