Optimal antithrombotic pharmacotherapy in patients affected by diabetes mellitus (DM) undergoing percutaneous coronary intervention is unclear. We sought to evaluate the safety and efficacy of bivalirudin compared with heparin plus a glycoprotein IIb/IIIa inhibitor (GPI) in patients with DM undergoing percutaneous coronary intervention. We pooled patient-level data from the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events-2, Acute Catheterization and Urgent Intervention Triage strategy, and Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction trials. The primary efficacy end point was the incidence of major adverse cardiac events, defined as the composite of death, myocardial infarction, or unplanned revascularization at 30 days. The primary safety end point was the incidence of 30-day non–coronary artery bypass graft-related major bleeding. All-cause mortality was reported at 30 days and 1 year. Of the 14,737 patients included in the pooled database, 3,641 (24.7%) had DM. Patients with DM had higher rates of 30-day major bleeding and 30-day and 1-year all-cause mortality. There were no differences in 30-day major adverse cardiac events between bivalirudin versus heparin plus GPI in patients with DM (6.9% vs 7.8%; relative risk [RR] 0.89, 95% CI 0.71 to 1.12) or without DM (7.5% vs 6.7%; RR 1.11, 95% CI 0.97 to 1.27; p interaction = 0.10). Bivalirudin treatment was associated with reduced risk of major bleeding in similar magnitude in patients with DM (4.3% vs 6.6% RR 0.68, 95% CI 0.51 to 0.89) or without DM (3.2% vs 6.1%; RR 0.51, 95% CI 0.43 to 0.61; p interaction = 0.15). The hemorrhagic benefit of bivalirudin was noted for both access site- and non–access site-related bleeding. Overall, bivalirudin treatment was associated with a significant 1-year mortality benefit (2.7% vs 3.3%; RR 0.82, 95% CI 0.68 to 0.98; p = 0.03), which was consistent between patients with or without DM (p interaction = 0.30). In conclusion, compared with heparin plus GPI, bivalirudin was associated with similar 30-day antithrombotic efficacy and better 30-day freedom from bleeding and 1-year mortality, irrespective of diabetic status.
Patients with diabetes mellitus (DM) and coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) are at a higher risk for mortality and adverse outcomes, including need for repeated revascularization, myocardial infarction (MI), stent thrombosis, and death. The optimal antithrombotic therapy in patients with DM undergoing PCI is still uncertain. Hyperglycemia is known to be associated with enhanced thrombin formation, platelet activation, and fibrin clot lysis resistance in acute coronary syndromes. Current European Society of Cardiology and American College of Cardiology/American Heart Association guidelines do not provide clear recommendations regarding antithrombotic pharmacotherapy in patients affected by DM. Although early trials suggested significant ischemic and mortality benefit in patients with DM undergoing PCI with glycoprotein IIb/IIIa inhibitors (GPIs), recent studies performed on a background of oral thienopyridines failed to replicate those benefits. Importantly, a drawback of routine GPI use that cannot be overlooked is the increase in hemorrhagic risk, which can have a substantial impact on morbidity and mortality. Bivalirudin is a direct thrombin inhibitor that is associated with similar antithrombotic efficacy and improved bleeding safety compared with standard therapies in patients undergoing PCI across the clinical spectrum of CAD. Due to the relatively low proportion of patients with DM enrolled in randomized controlled trials (RCTs) evaluating intraprocedural antithrombotic strategies, the safety and efficacy of bivalirudin in patients with DM undergoing PCI remains unclear. Therefore, in the present patient-level pooled analysis of RCTs, we sought to investigate with enhanced statistical power the safety and efficacy of bivalirudin monotherapy versus heparin plus GPI in patients with DM undergoing PCI.
Methods
We pooled patient-level data from 3 RCTs evaluating the safety and efficacy of bivalirudin therapy versus heparin plus routine use of GPI in patients undergoing PCI. The study designs, major inclusion and exclusion criteria, end points, definitions, and results for each individual study have been previously reported. Briefly, the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events-2 trial was a study of 6,010 patients undergoing elective or urgent PCI for stable angina, inducible ischemia, or unstable ischemic syndromes. Patients were randomized either to heparin (unfractionated heparin; 65 IU/kg bolus) plus a GPI (abciximab or eptifibatide) or bivalirudin (0.75 mg/kg bolus before PCI with 1.75 mg/kg/hour infusion during procedure) with provisional GPI, which was used in ≈7% of patients. Provisional GPI with either abciximab or eptifibatide could be administered for procedural or angiographic complications in the bivalirudin group. Most of the patients received aspirin and clopidogrel pretreatment, with subsequent daily administration for at least 30 days after intervention. The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial was a study of 13,819 patients with moderate- and high-risk acute coronary syndromes undergoing an early invasive strategy and triaged after angiography to medical therapy or revascularization by either PCI or coronary artery bypass graft. Of these, 7,789 patients underwent PCI. Patients were randomized to either heparin (unfractionated heparin or enoxaparin) plus a GPI, bivalirudin with planned GPI, or bivalirudin with provisional GPI. Bivalirudin plus planned GPI comprised ≈50% of the patients treated with bivalirudin. Unfractionated heparin was administered as an intravenous bolus of 60 IU/kg with 12 IU/kg/hour infusion to target an activated partial thromboplastin time of 50 to 75 seconds before angiography and an activated clotting time of 200 to 250 seconds. Enoxaparin was administered subcutaneously 1 mg/kg twice a day before angiography; then, an intravenous bolus of an additional 0.3 mg/kg or 0.75 mg/kg was administered before PCI if the most recent subcutaneous dose had been given more than 8 hours or 16 hours earlier, respectively. The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial was a study of 3,602 patients presenting with ST-segment elevation myocardial infarction within 12 hours after symptom onset undergoing primary PCI. Patients were randomized to either heparin (unfractionated heparin; 60 IU/kg bolus with subsequent boluses targeting an activated clotting time of 200 to 250 seconds) with planned GPI therapy or bivalirudin with provisional GPI therapy. Provisional GPI with either abciximab or eptifibatide was permitted at the discretion of the operators and was used in ≈7% of patients treated with bivalirudin. In both the ACUITY and HORIZONS-AMI trials, all patients received aspirin and a thienopyridine (clopidogrel or ticlopidine) before and after PCI (lifelong aspirin and thienopyridine recommended for at least 1 year).
The objectives of the present patient-level pooled analysis were: (1) to investigate the impact of DM on outcomes in patients undergoing PCI across the clinical spectrum of CAD and (2) to evaluate the safety and efficacy of bivalirudin monotherapy compared with heparin plus routine use of GPI according to DM status. The primary efficacy end point was the rate of major adverse cardiac events (MACE) defined as the composite of death, MI, and unplanned revascularization at 30 days after PCI. The primary safety end point was the incidence of 30-day non–coronary artery bypass graft-related major bleeding. The main secondary end point was all-cause mortality at 30 days and 1 year after the index procedure. Major bleeding was defined as the occurrence of intracranial or intraocular hemorrhage or a decrease in the hemoglobin level of ≥4 g/dl without an overt bleeding source or ≥3 g/dl with an overt bleeding source. In addition, the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events-2 major bleeding definition also included retroperitoneal bleeds and transfusions requiring 2 or more units of packed red blood cells or whole blood. HORIZONS-AMI and ACUITY included access site bleeding requiring intervention or a hematoma that was ≥5 cm in diameter, blood transfusions, and operation for bleeding in their major bleeding definitions. Major end points were adjudicated by blinded independent core laboratories or by clinical event adjudication committees.
Categorical variables were compared with the Cochran–Mantel–Haenszel test and stratified by trial. Continuous variables are presented as mean ± SD and were compared using the Wilcoxon 2-sample test. All p values are 2-sided. Thirty-day and 1-year event rates were estimated by the Kaplan–Meier method and compared with the log-rank test. Interaction testing was performed to assess the treatment effect consistency between DM status and antithrombotic therapy on 30-day outcomes and 1-year mortality. Treatment effect consistency for the primary efficacy and safety end points were performed across specific subgroups of interest within the DM cohort. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina). A p value <0.05 was considered statistically significant.
Results
Of the 14,737 patients included in the pooled data set, 3,641 had DM (24.7%). Of those, 628 (17.2%) were insulin treated. Baseline characteristics in patients with or without DM are reported in Table 1 . Patients with DM were older, were more commonly women, and were affected by multiple co-morbidities. Procedural characteristics are presented in Supplementary Table 1 . Patients with DM more commonly had features of higher procedural complexity, with more lesions and vessel treated. In particular, patients with DM had greater median Syntax score compared with the non-DM group (ACUITY trial PCI cohort only [n = 2,614]; 10.0 [interquartile range: 6.0 to 17.0] vs 9.0 [interquartile range: 5.0 to 15.5]; p = 0.002). Thirty-day and 1-year outcomes in patients with or without DM are reported in Table 2 . Patients with DM had higher rates of 30-day major bleeding and mortality at 30 days and 1 year although there were no differences in the incidence of 30-day MACE between the 2 groups.
| Variable | Diabetes Mellitus | p-value | |
|---|---|---|---|
| No (n = 11,096) | Yes (n = 3,641) | ||
| Age (years) | 62 [53, 71] | 64 [56, 72] | <0.0001 |
| Men | 8,457 (76.2%) | 2,510 (68.9%) | <0.0001 |
| Insulin-dependent diabetes mellitus | — | 628 (17.2%) | — |
| Current smoker | 3,933 (35.8%) | 825 (22.7%) | <0.0001 |
| Hypertension | 6,362 (57.5%) | 2,925 (80.3%) | <0.0001 |
| Cerebrovascular disease | 286 (3.6%) | 160 (5.3%) | <0.0001 |
| Previous myocardial infarction | 2,920 (26.6%) | 1205 (33.8%) | <0.0001 |
| Previous coronary artery bypass graft | 1,355 (12.2%) | 756 (20.8%) | <0.0001 |
| Previous percutaneous coronary intervention | 3,068 (27.7%) | 1,399 (38.7%) | <0.0001 |
| Clinical presentation | <0.0001 | ||
| Stable angina pectoris | 1,089 (9.8%) | 400 (11.0%) | |
| Unstable angina pectoris or NSTEMI | 5,601 (50.5%) | 2,109 (57.9%) | |
| STEMI | 3,006 (27.1%) | 593 (16.3%) | |
| Other | 1,400 (12.6%) | 539 (14.8%) | |
| Chronic kidney disease ∗ | 88.4 [68, 112] | 90 [66, 118] | 0.02 |
| Hemoglobin (g/dL) | 14.2 [13, 15] | 13.7 [13, 15] | <0.0001 |
| White blood cell count (×10 3 /mm 3 ) | 8.1 [7, 11] | 7.8 [6, 10] | <0.0001 |
| Platelet count (×10 3 /mm 3 ) | 231 [194, 275] | 224 [186, 270] | <0.0001 |
| Diabetes Mellitus | p-value | ||
|---|---|---|---|
| No (n = 11,096) | Yes (n = 3,641) | ||
| 30-Day outcomes | |||
| Major adverse cardiac events | 790 (7.1%) | 267 (7.3%) | 0.82 |
| All-cause mortality or myocardial infarction | 668 (6.0%) | 228 (6.3%) | 0.93 |
| All-cause mortality | 116 (1.0%) | 47 (1.3%) | 0.02 |
| Myocardial infarction | 581 (5.2%) | 188 (5.2%) | 0.19 |
| Unplanned revascularization | 238 (2.1%) | 86 (2.4%) | 0.45 |
| Non-coronary artery bypass graft major bleeding | 514 (4.6%) | 198 (5.4%) | 0.005 |
| Non-coronary artery bypass graft minor bleeding | 1,651 (20.4%) | 569 (18.7%) | 0.04 |
| Net adverse clinical events | 1,183 (10.7%) | 418 (11.5%) | 0.19 |
| 1-Year outcomes | |||
| All-cause mortality | 278 (2.5%) | 158 (4.3%) | <0.0001 |
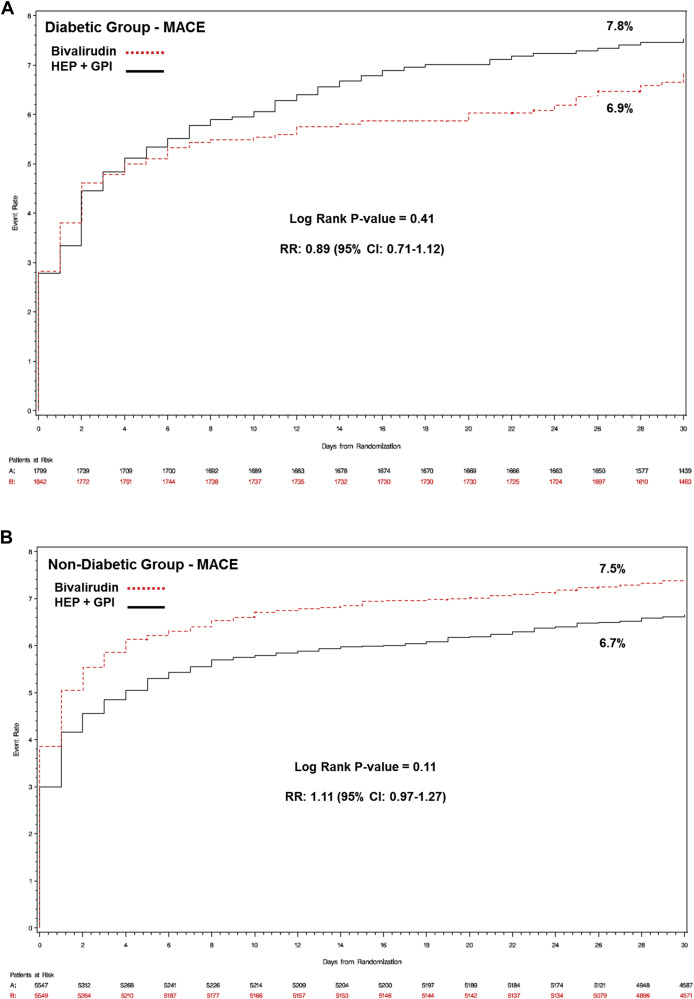
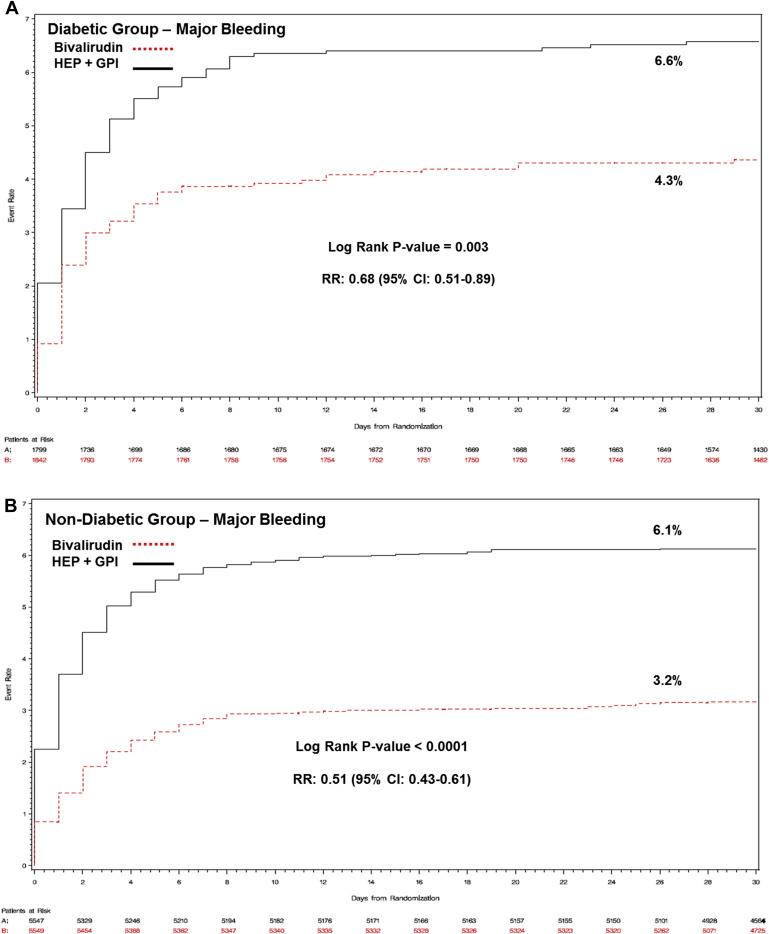
Baseline, procedural characteristics, and medications used in patients with or without DM according to the randomized antithrombotic treatment are reported in Table 3 , Supplementary Tables 2 and 3 , respectively. Thirty-day and 1-year outcomes are reported in Table 4 . At 30 days, there were no differences in the primary efficacy end point between bivalirudin and heparin plus GPI in patients with DM ( Figure 1 ) and without DM ( Figure 1 ), with a uniform treatment effect independent of diabetic status (p interaction = 0.10). Lower rates of major bleeding were observed in both patients with DM ( Figure 2 ) and without ( Figure 2 ) DM treated with bivalirudin, again, with a similar treatment effect between groups (p interaction = 0.15). The hemorrhagic benefit of bivalirudin was noted for both access site- and non–access site-related bleeding ( Table 5 ), with highly significant reductions in gastrointestinal bleeding rates in both DM and non-DM patients. Bivalirudin monotherapy was associated with a lower risk for the composite of death or MI at 30 days for patients with DM (5.8% vs 6.8%; relative risk [RR] 0.85, 95% CI 0.66 to 1.10); conversely, for patients without DM, bivalirudin monotherapy was associated with a higher rate of death or MI (6.5% vs 5.6%; RR 1.17, 95% CI 1.01 to 1.35), driven by excess MIs, with significant statistical heterogeneity (p interaction = 0.03).
| No Diabetes Mellitus | p-value | Diabetes Mellitus | p-value | |||
|---|---|---|---|---|---|---|
| Bivalirudin (n = 5,549) | Heparin + GPI (n = 5,547) | Bivalirudin (n = 1,842) | Heparin + GPI (n = 1,799) | |||
| Study | ||||||
| HORIZONS-AMI | 1,518 (27.4%) | 1,488 (26.8%) | 0.16 | 281 (15.3%) | 312 (17.3%) | 0.16 |
| ACUITY | 1,882 (33.9%) | 1,840 (33.2%) | 0.96 | 721 (39.1%) | 703 (39.1%) | 0.96 |
| REPLACE-2 | 2,149 (38.7%) | 2,219 (40.0%) | 0.08 | 840 (45.6%) | 784 (43.6%) | 0.08 |
| Age (years) | 61.7 [53, 70] | 62 [53, 71] | 0.57 | 64 [56, 72] | 65 [57, 72] | 0.03 |
| Men | 4,247 (76.5%) | 4,210 (75.9%) | 0.42 | 1,278 (69.4%) | 1,232 (68.5%) | 0.55 |
| Insulin-dependent diabetes mellitus | — | — | — | 313 (17.2%) | 315 (17.8%) | 0.10 |
| Current smoker | 2,023 (36.8%) | 1,910 (34.8%) | 0.03 | 405 (22.3%) | 420 (23.6%) | 0.32 |
| Hypertension | 3,131 (56.6%) | 3,231 (58.4%) | 0.06 | 1,465 (79.7%) | 1,460 (81.3%) | 0.21 |
| Cerebrovascular events | 153 (3.8%) | 133 (3.3%) | 0.21 | 92 (5.9%) | 68 (4.6%) | 0.10 |
| Previous myocardial infarction | 1,453 (26.5%) | 1,467 (26.8%) | 0.77 | 627 (34.8%) | 578 (32.8%) | 0.20 |
| Previous coronary artery bypass graft | 678 (12.2%) | 677 (12.2%) | 0.98 | 385 (20.9%) | 371 (20.7%) | 0.83 |
| Previous percutaneous coronary intervention | 1,514 (27.4%) | 1,554 (28.1%) | 0.40 | 724 (39.7%) | 675 (37.8%) | 0.24 |
| Clinical presentation | 0.24 | 0.22 | ||||
| Stable angina pectoris | 548 (9.9%) | 541 (9.8%) | 220 (11.9%) | 180 (10.0%) | ||
| Unstable angina pectoris | 1,392 (25.1%) | 1,459 (26.3%) | 613 (33.3%) | 598 (33.2%) | ||
| NSTEMI | 1,413 (25.5%) | 1,337 (24.1%) | 452 (24.5%) | 446 (24.8%) | ||
| STEMI | 1,518 (27.4%) | 1,488 (26.8%) | 281 (15.3%) | 312 (17.3%) | ||
| Other | 678 (12.2%) | 722 (13.0%) | 276 (15.0%) | 263 (14.6%) | ||
| Chronic kidney disease ∗ | 854 (16.1%) | 872 (16.4%) | 0.67 | 299 (16.9%) | 343 (20.0%) | 0.01 |
| Hemoglobin (g/dL) | 14.3 [13, 15] | 14.2 [13, 15] | 0.04 | 13.8 [13, 15] | 13.6 [13, 15] | 0.07 |
| White blood cell count (×10 3 /mm 3 ) | 8.2 [7, 11] | 8.1 [6, 11] | 0.09 | 7.9 [6, 10] | 7.8 [6, 10] | 0.59 |
| Platelet count (×10 3 /mm 3 ) | 231 [193, 275] | 231 [195, 276] | 0.71 | 225 [186, 268] | 222 [186, 268] | 0.43 |
| No Diabetes Mellitus | Relative Risk (95% CI) | Diabetes Mellitus | Relative Risk (95% CI) | P interaction | |||
|---|---|---|---|---|---|---|---|
| Bivalirudin (n = 5549) | Heparin + GPI (n = 5547) | Bivalirudin (n = 1842) | Heparin + GPI (n = 1799) | ||||
| 30-Day outcomes | |||||||
| Major adverse cardiac events | 416 (7.5%) | 374 (6.7%) | 1.11 (0.97–1.27) | 127 (6.9%) | 140 (7.8%) | 0.89 (0.71–1.12) | 0.10 |
| All-cause mortality or myocardial infarction | 359 (6.5%) | 309 (5.6%) | 1.17 (1.01–1.35) | 106 (5.8%) | 122 (6.8%) | 0.85 (0.66–1.10) | 0.03 |
| All-cause mortality | 54 (1.0%) | 62 (1.1%) | 0.86 (0.60–1.23) | 18 (1.0%) | 29 (1.6%) | 0.65 (0.36–1.16) | 0.30 |
| Myocardial infarction | 317 (5.7%) | 264 (4.8%) | 1.21 (1.03–1.42) | 92 (5.0%) | 96 (5.3%) | 0.92 (0.70–1.22) | 0.13 |
| Unplanned revascularization | 121 (2.2%) | 117 (2.1%) | 1.03 (0.80–1.32) | 44 (2.4%) | 42 (2.3%) | 1.04 (0.69–1.57) | 0.97 |
| Non-CABG major bleeding | 175 (3.2%) | 339 (6.1%) | 0.51 (0.43–0.61) | 80 (4.3%) | 118 (6.6%) | 0.68 (0.51–0.89) | 0.15 |
| Non-CABG minor bleeding | 583 (14.5%) | 1068 (26.3%) | 0.55 (0.50–0.59) | 50 (2.7%) | 89 (4.9%) | 0.56 (0.48–0.65) | 0.66 |
| Net adverse clinical events | 543 (9.8%) | 640 (11.5%) | 0.85 (0.76–0.94) | 193 (10.5%) | 225 (12.5%) | 0.85 (0.71–1.01) | 0.90 |
| 1-Year outcomes | |||||||
| All-cause mortality | 130 (2.3%) | 148 (2.7%) | 0.87 (0.69–1.10) | 67 (3.6%) | 91 (5.1%) | 0.73 (0.54–0.99) | 0.30 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


