Rupture EVAR
MEHDI J. TEYMOURI, PHILIP K. PATY, PHILIP S. K. PATY, and MANISH MEHTA
Presentation
A 69-year-old man presents to the emergency room with sudden onset of abdominal pain and near syncope. His past medical history is significant for hypertension, coronary artery disease, hyperlipidemia, and smoking. On presentation to the emergency room, the patient has complaints of abdominal pain, is diaphoretic, and is hypotensive (BP: 82/50 mm Hg). He denies any prior similar episodes and denies any chest or back pain. Physical examination does not indicate any murmurs, pulses are faint and normal, abdomen is distended with a pulsatile mass, femoral pulses are intact, and his feet appear well perfused.
Differential Diagnosis
Patients with a ruptured abdominal aortic aneurysm (r-AAA) typically present with abdominal and/or back pain, hypotension with or without syncope, and a pulsatile abdominal mass. The presence of all three findings is considered diagnostic for r-AAA. Rupture of AAA remains a lethal condition and had an expected overall mortality of nearly 50% nearly a decade ago. Over the past decade, with the evolution of endovascular aneurysm repair (EVAR) from elective to emergent r-AAA has had a significant impact in improving patient survival with most studies reporting an expected rupture EVAR mortality in proximity of 20%. The implications of improvements in our technical ability to offer rupture EVAR are significant: To date, no other therapy has offered such a survival advantage to these high-risk patients presenting with r-AAA.
Workup
An emergency room FAST exam (Focused Assessment with Sonography in Trauma) can be extremely helpful in making the diagnosis of r-AAA. The hemodynamic status of the ruptured AAA patient generally dictates the need for a preoperative CT scan, and although while planning for this emergent open surgical repair, a preoperative CT is not considered a necessity, while planning an emergent EVAR, its critically important to obtain a preoperative CT scan for evaluating the feasibility of EVAR as well as for stent graft sizing. Adequate resuscitation of patients with r-AAA is vital to a successful outcome. As long as the patients maintain a measurable blood pressure, the techniques of “permissive hypotension” by limiting the resuscitation to maintain a detectable blood pressure can help minimize ongoing hemorrhage.
When considering these endovascular techniques for treating r-AAA, one has to prepare for the challenges of streamlining patient care from the emergency room (ER) to the operating room (OR) and the subsequent endovascular procedure that often requires a multidisciplinary approach and a change in paradigm and local cultures. Furthermore, there is a need for adequate fluoroscopic equipment, trained personnel, and a variety of available stent graft, and ancillary equipment.
Collectively, worldwide experience demonstrates that an increasing number of r-AAAs are being treated by EVAR. The endovascular approach is less invasive, eliminates laparotomy, eliminates aortic cross clamping, decreases surgical bleeding and possibly general anesthesia, and has been shown to decrease the mortality of r-AAA repair with fewer complications, shorter hospital length of stay, and more patients being able to return home rather than going to institutional care after these emergent procedures. An understanding of the context in which the r-AAA patients were treated, the underlying patient selection, and the local, regional, and national influences in offering endovascular treatments to r-AAA patients is of critical importance when evaluating the evidence in favor of or against of r-EVAR.
Endovascular Approach
There remain several fundamental concerns regarding EVAR for ruptured AAA that include anatomical suitability for EVAR, the availability of dedicated staff and equipment to perform emergent EVAR at all hours, feasibility of treating hemodynamically stable and unstable patients by EVAR, and the surgeon/interventionist’s ability to manage unexpected scenarios under emergent circumstances. Many of the high-volume institutions have adopted a standardized protocol-based approach that includes a heightened awareness among the ER staff to suspect the diagnosis of ruptured AAA and notify the on-call vascular surgeon and the OR staff.
Since not all patients with ruptured AAA can undergo endovascular repair, all OR/hybrid endovascular OR suits should be setup to facilitate EVAR, as well as open surgical repair. Depending on the size of the room and the fluoroscopic equipment that can be fixed or portable with viewing screens and power injectors, one has to customize the layout of the OR suite that is conducive for endovascular and open surgical repair; we have found it best to set up the room for endovascular repair with standard needles, wires, catheters, and sheaths open on a sterile table, have the surgical instruments in the room if needed, situate the patient on the OR table, and as the anesthesiology team prepares the patient, set up the fluoroscopic equipment and supplies (Table 1).
TABLE 1. Rupture EVAR

The patient is prepped and draped in supine position. Via a percutaneous or femoral artery cut-down, ipsilateral access is obtained using a needle, floppy guidewire, and a guiding catheter. The floppy guidewire is exchanged for a super-stiff wire that can be used to place a large sheath (12 –14 French × 45 cm length) in the ipsilateral femoral artery and the sheath advanced up to the juxtarenal abdominal aorta, so it is ready to be used to deliver and support the aortic occlusion balloon (AOB) if needed. A compliant occlusion balloon should always be available in these procedures, and in hemodynamically unstable patients, the occlusion balloon is advanced through the ipsilateral sheath over the super-stiff wire into the supraceliac abdominal aorta under fluoroscopic guidance, and the balloon is inflated as needed. Contralateral femoral access is subsequently obtained via percutaneous or cut-down approach in a similar fashion and a “marker flush-catheter” advanced to the juxtarenal aorta for an arteriogram.
The placement of the stent graft main body is planned based on the aortoiliac morphology that is best suited for EVAR. Unless prohibitive, in hemodynamically stable patients, following the initial arteriogram, the AOB is removed from the initial ipsilateral side and the stent graft main body advanced under fluoroscopic guidance; this limits the number of catheter exchanges. In hemodynamically unstable patients who require inflation of the AOB, the “marker flush-catheter” is exchanged for the stent graft main body that is delivered up to the aortic neck. An arteriogram is done via the sheath that is used to support the AOB, the tip of the stent graft main body is aligned with the lowermost renal artery, the occlusion balloon is subsequently deflated and withdrawn back with the delivery sheath into the AAA, and the stent graft main body deployed. The remainder of the EVAR procedure is performed similar to as in elective circumstances: (1) the tip of the stent graft main body aligned with the lowermost renal artery, (2) the contralateral gate aligned to facilitate expeditious “gate cannulation,” and (3) the ipsilateral and contralateral iliac extensions planned and deployed as needed.
Special Intraoperative Considerations
The proportion of r-AAA patients that are suitable for EVAR is variable and on the basis of two meta-analysis ranges from 47% to 67%. r-EVAR patients with hostile aortic necks had a significantly higher incidence of female gender, mean maximum AAA diameter, abdominal compartment syndrome (ACS), Type I endoleaks, and the need for all secondary interventions.
Depending on one’s comfort level and the logistics, EVAR for rupture can be performed under local anesthesia via percutaneous approach to general anesthesia and femoral artery cut-down. The potential benefits of local anesthesia/conscious sedation and percutaneous approach is that it might avoid the loss of “sympathetic tone” in the compromised ruptured AAA patients. One has to be comfortable with obtaining percutaneous access and using closure devices in patients who might be hemodynamically unstable with difficult to palpate femoral pulses. Although the advantage may be significant, it must be balanced by the potential difficulties encountered during these emergent procedures, as the patient might not be coherent and cooperative enough to lie still.
The appropriate use of AOBs in hemodynamically unstable patients is vital to the success of EVAR. Access for AOBs can be obtained via the brachial or the femoral artery. There are several advantages of the femoral approach: (1) it allows the anesthesia team to have access to both upper extremities for arterial and venous access, (2) the patients who require the AOB are often hypotensive, and, in these patients, percutaneous brachial access can be more cumbersome and time consuming than femoral cut-down, and (3) the currently available AOBs require at least a 12–14 French sheath, which requires a brachial artery cut-down and repair, and stiff wires and catheters across the aortic arch without prior imaging under emergent circumstances might lead to other arterial injuries and/or embolization causing stroke.
Several important points should be considered during placement of the AOB. The sheath supporting the balloon should be advanced and supported fully into the aortic neck prior to inflation of the occlusion balloon as this will prevent downward displacement and prolapse of the occlusion balloon into the AAA (Fig. 1). Inability to fully engage the sheath into the aortic neck due to the presence of significant aortoiliac stenosis, calcifications, or tortuosity might result in downward displacement of the inflated occlusion balloon; this often required forward traction on the inflated balloon catheter to maintain adequate position at the suprarenal/supraceliac aorta (Fig. 2A–C). If inflation of the aortic balloon is required to maintain a viable blood pressure, just prior to deployment of the stent graft main body, the AOB should be deflated from the suprarenal level and withdrawn. The stent graft main body is subsequently deployed; this will avoid trapping the compliant AOB between the aortic neck and the stent graft. This temporary deflation of the AOB rarely results in hemodynamic collapse and usually is of little consequence. In hemodynamically unstable patients, the occlusion balloon can be redirected into the aortic neck from the side ipsilateral to the stent graft main body and reinflated at the infrarenal aortic neck within the stent graft main body; this allows for aortic occlusion and does not interfere with the remainder of the endovascular procedure (Fig. 3A–E).
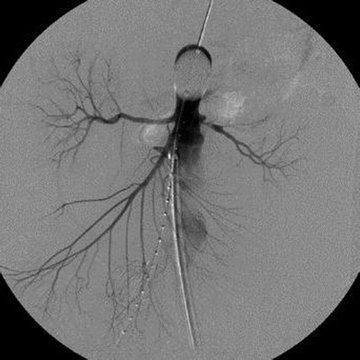
FIGURE 1 The sheath supporting the AOB should be advanced and supported fully into the aortic neck to prevent downward displacement and prolapse of the occlusion balloon into the abdominal aortic aneurysm. (From Mehta M. Endovascular aneurysm repair for ruptured abdominal aortic aneurysm: the Albany Vascular Group approach. J Vasc Surg. 2010;52(6):1706–1712.)



