Fig. 5.1
Checking the intubation depth using bronchofiberscope
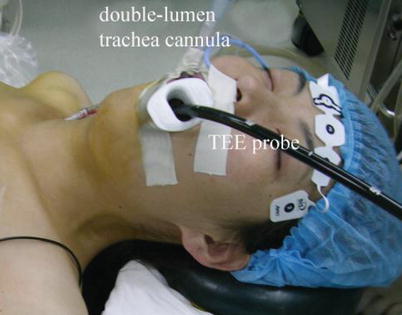
Fig. 5.2
Intraoperative TEE check
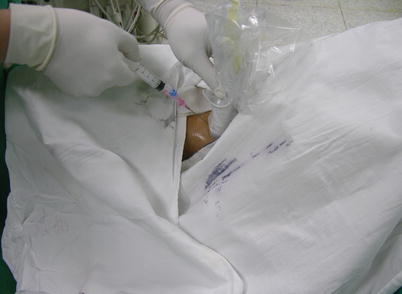
Fig. 5.3
Puncture the internal jugular vein under TEE guidance
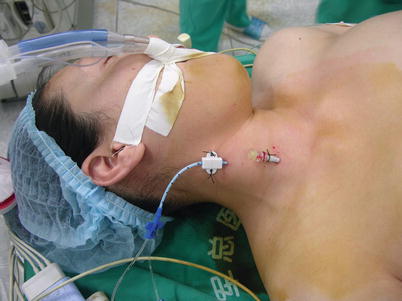
Fig. 5.4
Central venous catheter and internal venous drainage cannula
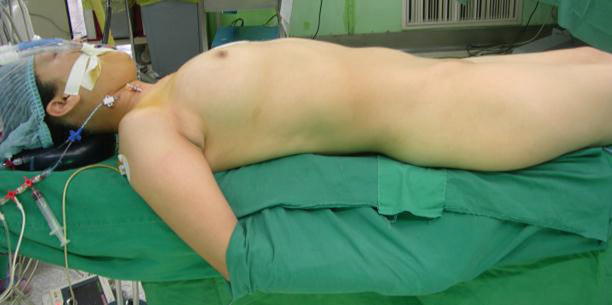
Fig. 5.5
The patient position
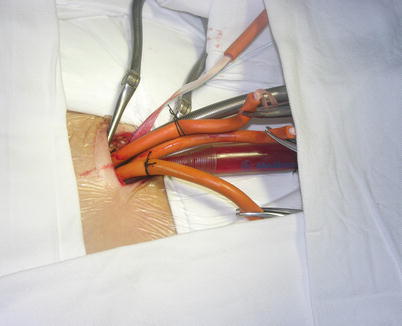
Fig. 5.6
Femoral arterial and venous cannulations
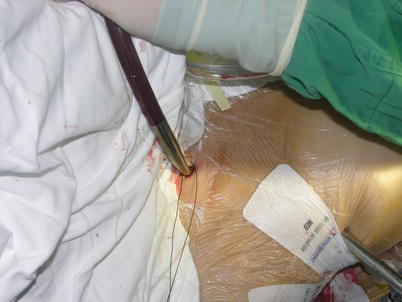
Fig. 5.7
Right internal jugular vein cannulation
5.1.2 Surgical Technique on Arrested Heart [14]
5.1.2.1 Robotic ASD Closure on Arrested Heart
After exclusion of the right lung, a 12-mm endoscopic trocar is placed into the right thoracic cavity through the fourth intercostal space (ICS), lateral to the nipple. The pleural space is insufflated with carbon dioxide at a maximum pressure of 8–10 mmHg, usually 6 mmHg, and the 30° endoscopic camera is inserted. A 1.5–2.0 cm incision is used as a working port in the same ICS for the patient-side surgeon. Additionally two 8-mm port incisions are made in the second and sixth ICS to allow insertion of the left and right instrument arms. The right instrument arm generally is positioned 4–6 cm lateral to the working port in the sixth ICS. The left instrument arm is positioned medial and cephalad to the right arm in the second or third ICS. The fourth arm trocar is placed in the midclavicular line in the 4th or 5th ICS [14], dependent on the patient’s rib direction and ICS. Two 16 F Angiocatheters are inserted in the 6th and 4th ICS laterally respectively for the placement of pericardial stay sutures (Figs. 5.8 and 5.9).
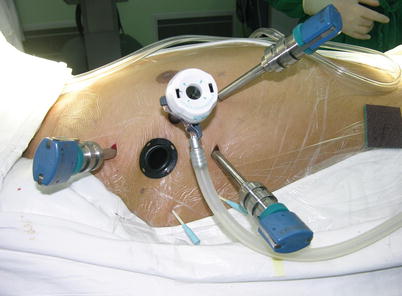
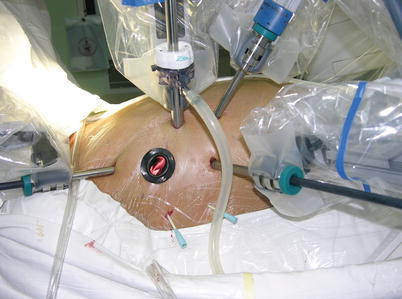

Fig. 5.8
Ports position

Fig. 5.9
Setup of the da Vinci Surgical System
CPB is initiated with kinetically assisted bicaval venous. The intrathoracic part of the operation began robotically with pericardiotomy and placement of pericardial stay sutures. The pericardium is opened longitudinally 1.5 cm anteriorly to the phrenic nerve (Fig. 5.10). The incision is extended superiorly to expose the superior vena cava and then extended inferiorly to the diaphragm to visualize the inferior vena cava. The pericardium stay sutures are placed on the right side of pericardium to rotate the heart for optimal exposure of the atrium (Fig. 5.11). And the 3rd pericardium stay suture is placed on the left superior side of pericardium through anterior chest (16 Ga Angio) to expose the aorta (Fig. 5.12).
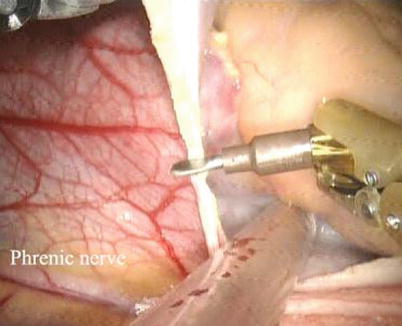
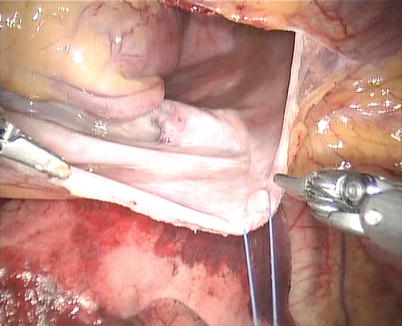
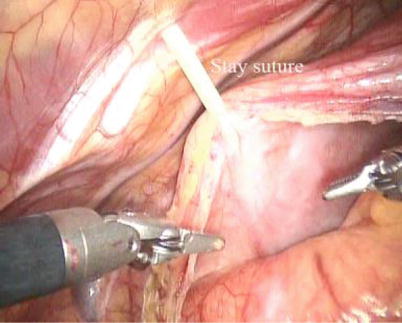

Fig. 5.10
The pericardium was opened anteriorly to the phrenic nerve

Fig. 5.11
The pericardium stay suture

Fig. 5.12
The stay suture on the left superior side of pericardium to expose the aorta
The space between the vena cavae and the pulmonary veins are dissected clear (Figs. 5.13 and 5.14). And the linear tapes are placed around the inferior and superior vena cavae (Figs. 5.15 and 5.16). The aortic occlusion is performed with a Chitwood cross-clamp via the midaxillary line in the fourth ICS (Figs. 5.17 and 5.18). Antegrade cold blood cardioplegic solution is administered directly through the anterior chest (the second ICS) with a 14 Ga Angiocatheter (Figs. 5.19 and 5.20).
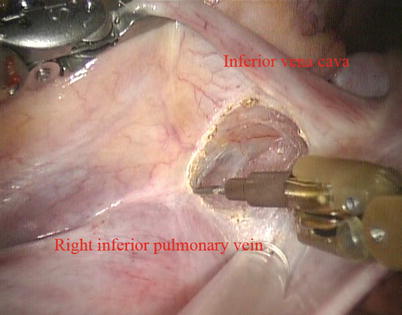
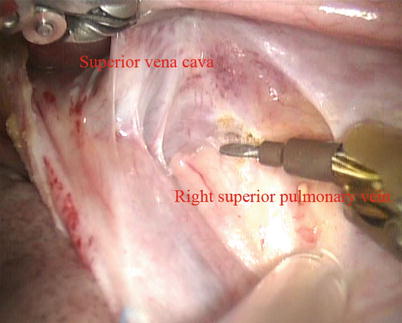
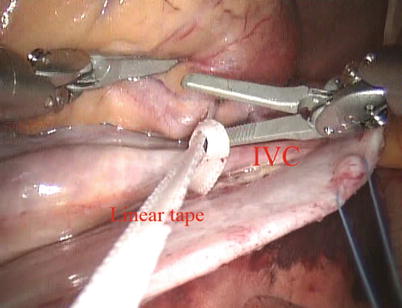
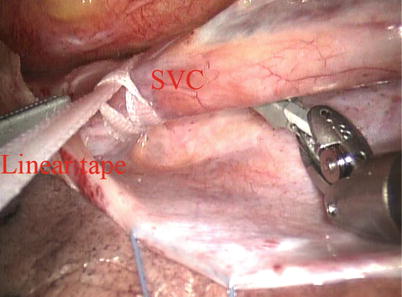
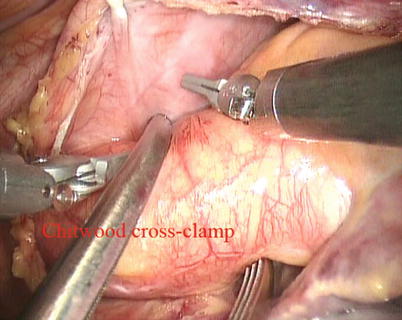
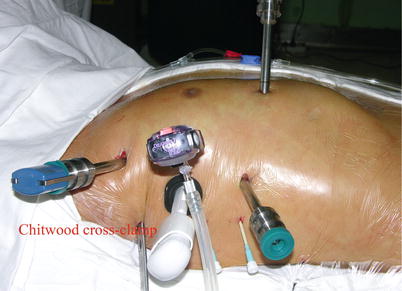
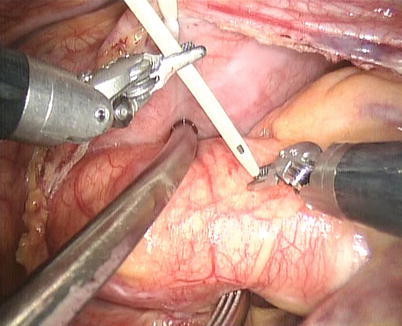
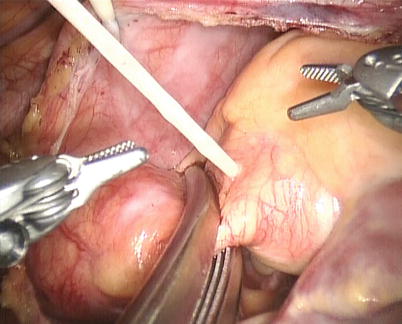

Fig. 5.13
Dissected the space between superior vena cava and right superior pulmonary vein

Fig. 5.14
Dissected the space between inferior vena cava and right inferior pulmonary vein

Fig. 5.15
The tape around the inferior vena cavae

Fig. 5.16
The tape around the superior vena cavae

Fig. 5.17
The extracorporeal position of Chitwood clamp

Fig. 5.18
The Chitwood cross-clamp

Fig. 5.19
14 Ga angiocatheter is inserted into the aorta

Fig. 5.20
Administration cold blood cardioplegic solution through the angiocatheter
After snaring of the superior and inferior vena cavae, the right atrium is opened (Fig. 5.21). The atrial retractor is introduced into the right atrium through the fourth robotic arm to expose the atrial septal defect (Fig. 5.22). Cardiotomy suction is passed through the working port by the patient-side surgeon. After thorough exploration, ASD is closed directly using 4-0 Gore-Tex running suture or autologous pericardial patching, depending on the size and location of ASD (Figs. 5.23 and 5.24). The knot tying is performed extracorporeally by patient-side surgeon using a knot pusher. And the atrial leakage is identified before closing right atrium. If the moderate to severe tricuspid valve regurgitation is diagnosed, the retractor is removed to expose the tricuspid valve and ring. The tricuspid valve plasty is performed using De Vaga technique. The result of tricuspid valve plasty is evaluated (Figs. 5.25 and 5.26). The right atrium is closed with double-layer 4-0 Gore-Tex running suture (Fig. 5.27). The deairing is done through the cardioplegia Angiocatheter. Then the aortic puncture site is closed using 4-0 Gore-Tex (Fig. 5.28). After adequate hemostasis achieved, the robotic arms are removed and a chest tube is inserted through right arm porthole. The right femoral artery is reconstructed after removing of the cannulas.
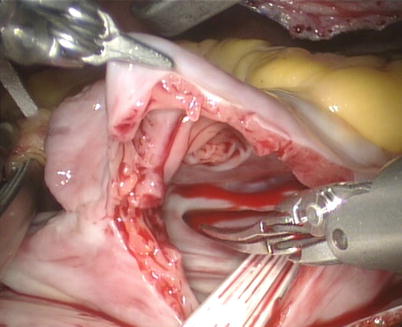
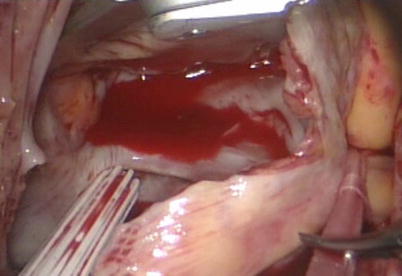
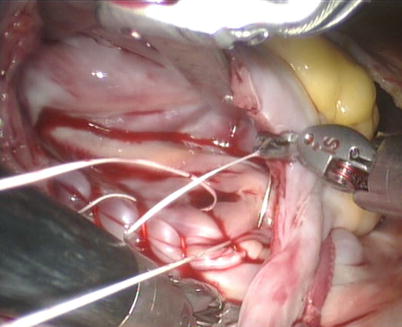
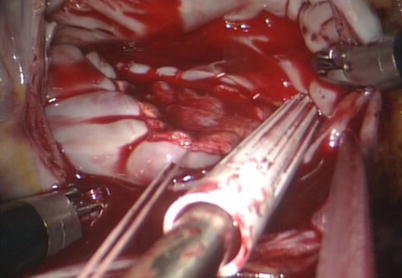
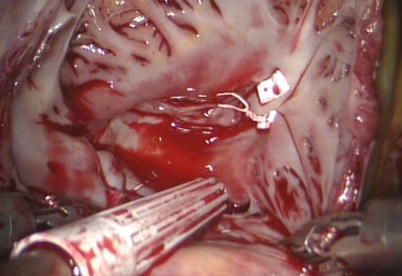
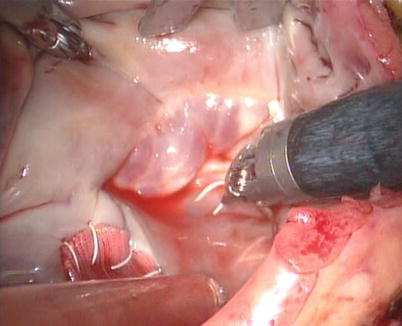
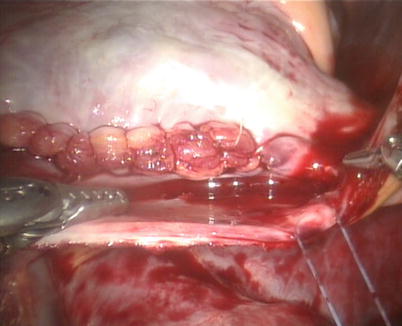
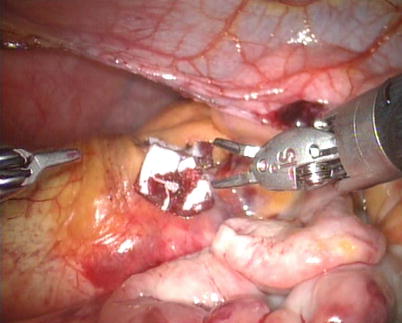

Fig. 5.21
The right atrium is opened

Fig. 5.22
The atrial retractor is introduced into right atrium

Fig. 5.23
Closing the ASD using running suture

Fig. 5.24
Closing the ASD using autologous pericardial patch

Fig. 5.25
Tricuspid valve plasty using De Vaga technique

Fig. 5.26
Evaluating the plasty result

Fig. 5.27
The right atrium is closed with double-layer running suture

Fig. 5.28
Aortic puncture site is closed
5.1.2.2 Robotic ASD Repair on Beating Heart
For ASD closure on beating heart, the aortic occlusion and cardioplegic solution administration can be avoided [15]. Under mild hypothermic conditions (rectal temperature, 34–35 °C), CPB full flow is maintained with mean systemic pressure more than 60 mmHg. To avoid air embolism, carbon dioxide with 6–8 mmHg is insufflated continuously into the chest for air displacement. On beating heart, a right atriotomy is performed under SVC and IVC snared with same techniques mentioned above, and ASD is exposed with atrial retractor by the 4th arm. A small suction catheter is placed in the right atrium, not in the left atrium through the working port. The direct closure with running suture or autologous pericardium patching can be used (Figs. 5.29, 5.30, 5.31, 5.32, and 5.33). As the inter-atrial septum is closed, the lung is briefly inflated, and the inter-atrial suture line is secured when there is no evidence of retained air in the left atrium.
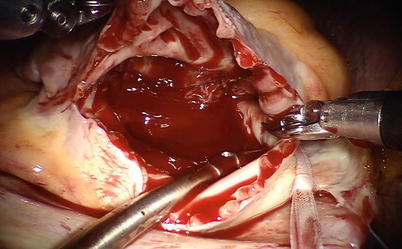
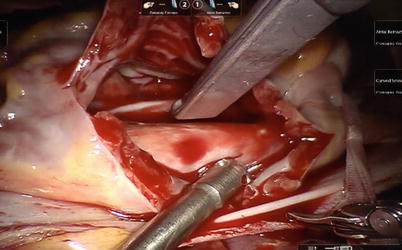
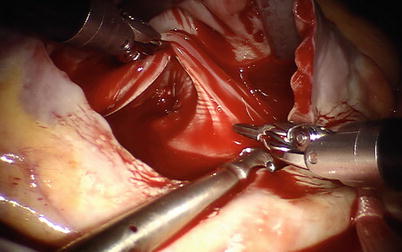
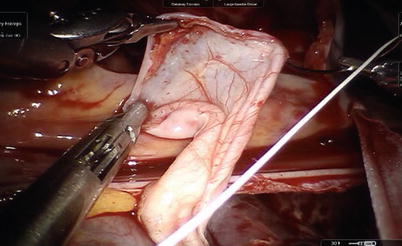
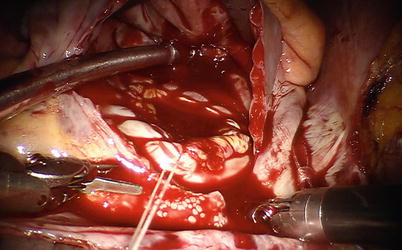

Fig. 5.29
The right atrium is opened on beating heart under SVC and IVC snared

Fig. 5.30
The atrial retractor is introduced into the right atrium

Fig. 5.31
ASD is exposed with the aid of atrial retractor

Fig. 5.32
The autologous pericardium patch is used for ASD closure

Fig. 5.33
Secured suture line when there is no evidence of retained air in the left atrium
Advantages of this method include avoidance of ischemia-reperfusion injury, performance of surgery in a more physiological state of the heart, decreased use of inotropic medications, and shorter hospital stay [16]. In addition, proximal aortic arteriosclerosis is the source of macro- and microemboli at the time of placement and release of the aortic cross-clamp. Potential concerns related to this technique may include performance of surgery in a relatively blood-filled field, limited surgical precision due to difficult exposure, risk of air embolization, and limited ability to perform very large ASD closure procedure on beating heart [16].
In fact, the operations can be performed without difficulty because the atrial retractor through 4th arm and small suction catheter from the working port could provide adequate visualization of the operative field. In addition, the concomitant surgery of tricuspid valve repair is easily performed. In our study, the complications related to beating heart ASD repair are not observed, such as, strokes or residual atrial septal defect due to dehiscence of the atrial suture line.
For the prevention of air embolism, each patient is positioned with the right chest elevated approximately 30°. Furthermore, the left atrium is kept full without suction in it during operation, and throughout the procedure carbon dioxide with 6–8 mmHg is insufflated continuously into the chest for air displacement. De-airing of left atrium at the end of the procedure is easily done.
Based on this knowledge, it seems reasonable to perform robotic beating-heart ASD closure surgery with on-pump beating heart conditions. This technique is simple and it shortens the duration of CPB and total operation. Besides, this technique does not increase the risk of central nervous system (CNS) injury. It is not possible to determine with certainty whether minor neuron-cognitive disorders due to microembolization occur. The neurocognitive evaluation is an important aspect that needs to be further investigated.
Possible contraindications to robotic beating-heart ASD repair may include the presence of mobile vegetations in patients with infective endocaditis or large left atrial thrombi, due to the risk of embolization. Inadequate experience of console surgeon certainly is contraindication to beating heart surgery.
5.1.3 Postoperative Management
Postoperation patients are monitored at the intensive care unit (ICU) and discharged to an intermediate care unit as soon as hemodynamics and spontaneous respiration has become adequately stabilized. Chest drains are removed when drainage reaches less than 50 ml/12 h. All patients undergo transthoracic echocardiography immediately before discharge from hospital and at 3 months after the procedure.
5.1.4 Surgical Results and Learning Curves
Totally endoscopic ASD closure remains a highly complex procedure, the performance of which requires experience with several non-routine operative steps, such as remote access perfusion and robotic cardiac surgery [17]. Moreover anesthesia management of those patients has additional non-routine steps as prerequisites, such as single-lung ventilation and advanced TEE for patient monitoring during remote access cardiopulmonary bypass (CPB) [13]. According to the previous literatures published, the robotic ASD closure is a more time-consuming operation compared with procedures in sternotomy, which requires long CPB and aortic occlusion time [10–13]. However, from our experience, we feel that robotic surgery is not time-consuming operation after overcoming the learning curve. The learning curves and operation times play a major role for the acceptance of such a program [13].
Between January 2007 and January 2013, 147 consecutive patients (99 female and 48 male) underwent ASD closure with da Vinci S or Si Surgical System at the PLA General Hospital. The mean age of the patients was 35.8 years old (12–65 years), and ostium secundum ASD was confirmed echocardiographically on all patients. Patients were excluded if they could not tolerate single-lung ventilation or peripheral CPB, or otherwise were considered poor candidates for a thoracoscopic approach. Fifty-four cases were completed on arrest heart (arrest heart group) from January 2007 to December 2008, and 93 cases on beating heart (beating heart group) from December 2008 to January 2013 (Table.5.1).
Table 5.1
The baseline characteristics of robotic ASD closure at the PLA General Hospital (01/2007 –01/2013)
Variables | Arrest heart group | Beating heart group | Both groups |
|---|---|---|---|
Total number of patients | 54 | 93 | 147 |
Gender | |||
Male (%) | 16(29.6) | 32(34.4) | 48(32.7) |
Female (%) | 38(70.3) | 61(65.6) | 99(67.3) |
Age (year) | 35.2 ± 13.1 | 36.4 ± 13.3 | 35.8 ± 12.3 |
Weight (kg) | 58.3 ± 10.0 | 61.7 ± 12.1 | 59.2 ± 13.6 |
Height (cm) | 162.6 ± 8.0 | 163.0 ± 8.2 | 162.7 ± 9.2 |
Pathology | |||
Atrial septal defect II, n (%) | 52(96.3) | 91(97.8) | 143(97.3) |
Patent foramen ovale, n (%) | 2(3.7) | 2(2.2) | 4(2.7) |
Median to sever tricuspid valve regurgitation, n (%) | 4(7.4) | 8(8.6) | 12(8.1) |
Median to sever pulmonary hypertension, n (%) | 6(11.1) | 9(9.7) | 15(10.2) |
Atrial septal aneurysm, n (%) | 4(7.4) | 2(2.2) | 6(4.1) |
Diameter of defect (cm) | 2.8 ± 1.6 | 2.7 ± 1.8 | 2.7 ± 2.0 |
Left ventricular ejection fraction (%) | 64.3 ± 7.1 | 65.8 ± 8.4 | 65.3 ± 6.4 |
Anesthesia and surgical techniques were described above. The direct closure of ASD was performed in 72 cases, and autologous pericardial patching in 75 cases, and 8 patients accepted ASD repair combined with tricuspid valve repair (Table 5.2). TEE confirmed complete closure and tricuspid valve plasty in all cases. Reoperations and intraoperative conversions to alternate procedures were not needed. Mortality and serious complications were not encountered.
Table 5.2




Results of robotic ASD closure at the PLA General Hospital (01/2007–01/2013)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


