Chapter 35 Risk Stratification after Acute ST-Segment Elevation and Non-ST-Segment Elevation Myocardial Infarction
INTRODUCTION
Each year in the United States alone, over 400,000 patients suffer an acute ST-segment elevation myocardial infarction (STEMI).1 Initial treatment of these patients has rapidly evolved over the past decade, particularly with the introduction of thrombolytic agents to achieve early coronary artery patency and the more selective use of primary percutaneous coronary intervention (PCI) for those admitted to hospitals with cardiac catheterization facilities. Along with these therapeutic advancements, there has been a complementary refinement in risk-stratification algorithms based on specific clinical criteria and the results of noninvasive imaging modalities. Nuclear cardiac imaging has retained an important role in the initial evaluation and risk stratification of patients surviving acute myocardial infarction (AMI), in guiding subsequent therapeutic decision making, and in monitoring the benefits of these therapeutic measures.
CLINICAL PREDICTORS OF RISK
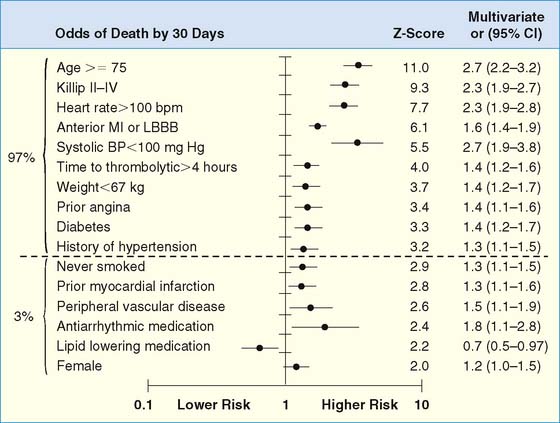
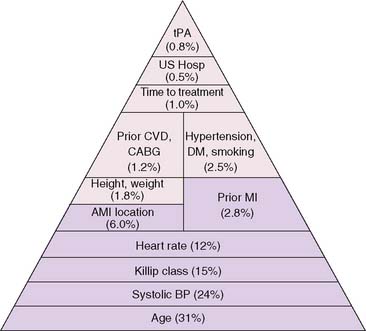
The development of clinical models from large patient cohorts has greatly enhanced risk stratification following STEMI. Data from the Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO)-I study, on 41,021 patients, identified age as the single most important predictor of 30-day mortality, followed by markers of myocardial dysfunction (i.e., lower systolic blood pressure, higher Killip class, elevated heart rate, and presence of anterior infarction).2 These five variables contained over 90% of the prognostic information derived from all recorded baseline clinical information (Fig. 35-1). Patients younger than age 60 years had a 2.4% 30-day mortality compared to a 20.5% mortality in those older than age 75. Mortality increased dramatically with increasing Killip class from I (5.1%) to IV (57.8%).
(From Ryan TJ, Antman EM, Brooks NH, et al: ACC/AHA guidelines for the management of patients with acute myocardial infarction: 1999 update: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee on Management of Acute Myocardial Infarction]. Available at www.acc.org. Adapted from ACC/AHA guidelines for the perioperative cardiovascular evaluation for noncardiac surgery, J Am Coll Cardiol 27: 910–948, 1996, with permission. Copyright © 1996 The American College of Cardiology and American Heart Association, Inc.)
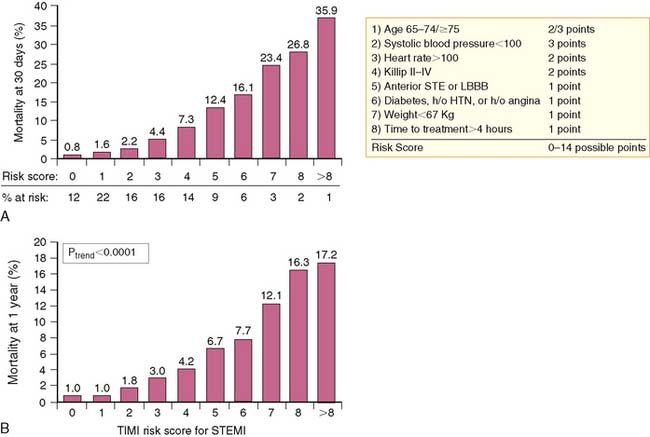
A simpler “bedside” prognostic tool was derived from the 14,114 patients enrolled in the Intravenous nPA for Treatment of Infarcting Myocardium Early (INTIME)-II trial.3 As observed from GUSTO-I, patient age older than 75 was the most important predictor of early (30-day) mortality, followed by hemodynamic instability, defined as increasing Killip class, heart rate over 100 beats/min, anterior AMI, and systolic hypotension (<100 mm Hg).3 Ten clinical variables accounted for 97% of all prognostic risk derived from the multivariate model and were selected for inclusion in the thrombolysis in myocardial infarction (TIMI) risk score (Fig. 35-2). The TIMI score predicted both 30-day and 1-year mortality (Fig. 35-3) and offered similar prognostic capability as the more complex multivariate model derived from GUSTO-I. The TIMI risk score was recently validated from 84,029 patients comprising the National Registry of Myocardial Infarction (NRMI) 3.4 The predictive behavior of the risk score was similar to the INTIME-II results among patients undergoing either coronary reperfusion with thrombolytic therapy or primary PCI. Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend coronary angiography in clinically high-risk, unstable patients.5
IMAGING PREDICTORS OF RISK
The most important predictors of subsequent patient outcome after AMI are infarct size,6–9 left ventricular (LV) ejection fraction (LVEF),6,10–12 LV volumes,13,14 and the presence and extent of residual myocardial ischemia.15–18 All of these variables can be directly determined through scintigraphic approaches.
Infarct Size
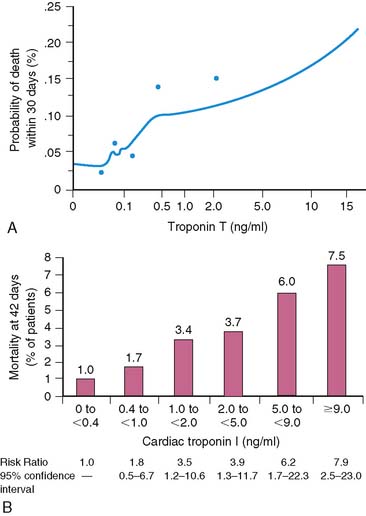
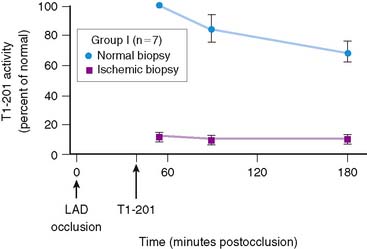
Serum levels of enzymatic markers accurately quantify infarct size and predict mortality (Fig. 35-4).8,9 Likewise, resting myocardial perfusion scintigraphy can accurately assess infarct size, since the uptake of thallium 201Tl and technetium 99mTc-based radiopharmaceuticals is primarily dependent on coronary blood flow and the presence of myocardial viability (see Chapter 28).19–21 In animal models of permanent coronary occlusion, initial 201Tl activity within the infarct zone correlates very well with coronary blood flow as measured by radiolabeled microspheres.22 However, the relative gradient in 201Tl activity between normal and infarcted myocardium decreases over time, as this isotope washes out from normal regions (Fig. 35-5). Due to the kinetics of 201Tl, the relative count activity within the infarct zone might appear to improve over time, simulating an artifactual reduction in scintigraphic infarct size.
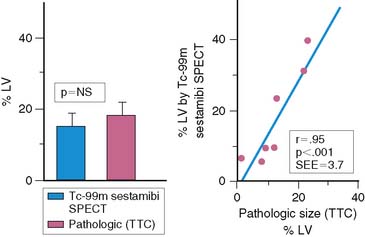
An alternative radiopharmaceutical for assessing infarct size is 99mTc sestamibi, since this isotope minimally redistributes once it is taken up by the myocardium.21,23–25 Unlike 201Tl, which can bidirectionally cross the cell membrane, sestamibi is actively taken up by mitochondria and trapped within the nucleus. Thus imaging can be performed even several hours after the initial resting injection. Animal investigations with sestamibi demonstrate close correlations between its initial uptake and occluded flows by microspheres, and the gradient in count activity between normally perfused and infarcted zones remains relatively constant over time.23,25 In animal models of coronary occlusion, there is a close correlation between scintigraphic and pathologic infarct size (Fig. 35-6) that is not influenced by the early reactive hyperemia observed following rapid reperfusion.23,25,26 These studies indicate that sestamibi imaging during AMI can estimate infarct size following coronary reperfusion independent of temporal restraints.
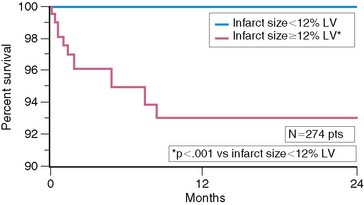
Scintigraphic infarct size predicts subsequent survival following AMI. In the Western Washington Study, resting 201Tl single-photon emission computed tomography (SPECT) was performed in 307 survivors of AMI a mean of 8.1 ± 6.4 weeks after enrollment.6 Patients with a quantified LV infarct size of 20% or greater had a significantly higher mortality rate than those with smaller infarcts (Fig. 35-7). Miller and colleagues prospectively followed 274 patients who received thrombolytic therapy for STEMI and had resting 99mTc sestamibi imaging a mean of 7 ± 8 days after hospital admission.7 The median LV infarct size for the entire cohort was 12% (range, 0% to 68%) with a 2-year mortality rate of 3%. Patients with an LV infarct size above the median had a significantly higher mortality rate compared to those with a smaller infarction (7% versus 0%, respectively; Fig. 35-8). This result is not unexpected, since infarct size and LVEF are inversely related.27 Infarct size as determined by resting 99mTc sestamibi SPECT is now commonly used as a surrogate marker for mortality in clinical trials assessing therapies during AMI.28–34
Left Ventricular Ejection Fraction
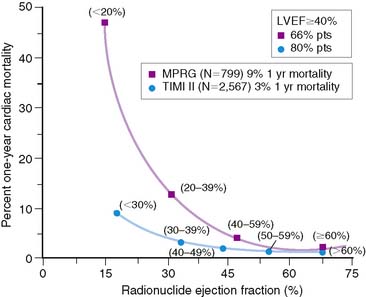
The LVEF remains the single best long-term predictor of mortality in survivors of AMI.6,10–12 Mortality rates are low in patients with an LVEF greater than 40% but rapidly increase with more severe LV dysfunction. In the Multicenter Post Infarction Research Group (MPRG)10 study, 799 survivors of AMI had gated radionuclide angiography following admission. The 1-year mortality rate for all patients was 9%, but 60% of all deaths occurred in the 33% with an LVEF less than 40%. A very high mortality rate of 47% was observed in the 3% of patients with an LVEF less than 20% (Fig. 35-9).
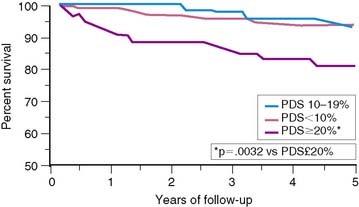
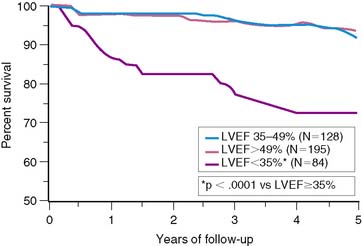
The important prognostic information obtained from LVEF has been confirmed in patients receiving reperfusion therapy during STEMI.6,11,35–37 The resultant LVEF and subsequent mortality rate are clearly dependent on the degree to which early coronary artery patency is achieved. In the GUSTO-I trial, LVEF and 30-day survival were both significantly higher in patients who achieved TIMI grade 3 flow (61% ± 14% [95.6%]) versus TIMI grade 0/1 (55% ± 14% [91.1%]) or TIMI grade 2 (56% ± 14% [92.6%]) flows, respectively.38 In the Survival and Ventricular Enlargement (SAVE) study, gated radionuclide angiography was performed within the first 2 weeks of AMI, and patients with an LVEF less than 40% were randomized to receive either placebo or captopril therapy.39 One-third of all patients had thrombolytic therapy during AMI. The mean LVEF in patients randomized to placebo was 31%, and the associated 1-year mortality approximately 12%—similar to the 15% mortality reported by the MPRG in patients with an LVEF less than 40%.10 Likewise, in the Western Washington Streptokinase Trials, LVEF measured 8.7 ± 6 weeks after enrollment was the best univariate and multivariate predictor of survival.6 In the 20% of patients with an LVEF less than 35%, the 1-year mortality was 15%, which increased to 22% by 3 years—virtually identical to the 22% placebo mortality reported in SAVE (Fig. 35-10).
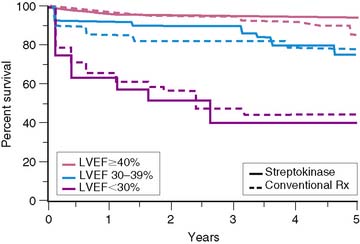
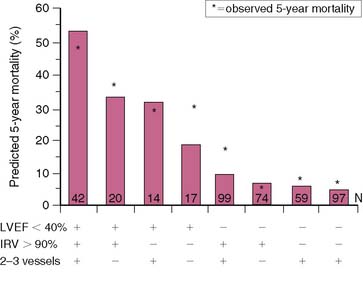
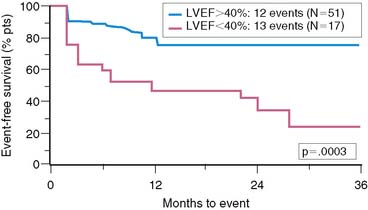
Similar results are reported by Simoons and coworkers in 422 patients randomized to intracoronary streptokinase versus placebo, where the LVEF was measured 10 to 40 days after AMI.11 In patients with LVEF greater than 40%, the 3-year mortality rate remained low (4.3%). Conversely, in patients with an LVEF less than 40%, the 1- and 3-year mortality rates increased with worsening LVEF, but irrespective of initial therapy (Fig. 35-11). Mortality was strongly influenced by LVEF and the extent of angiographic coronary artery disease (CAD) (Fig. 35-12), the latter presumably an indicator of the extent of jeopardized myocardium. In the series by Dakik and colleagues, the LVEF was the only significant predictor of infarct-free survival. The relative risk of death or nonfatal MI doubled for every 10% decrease in LVEF (RR = 2.06, 95% CI, 1.17–3.64; P = 0.01) (Fig. 35-13).35
The TIMI-II36 and Grupo Italiano por lo Studio della Streptochinase Nell’Infarcto Miocardico (GISSI)-237 trials indicate that survival at any given LVEF is better in patients who receive thrombolytic therapy compared to historical controls in the prethrombolytic era (see Fig. 35-9).10 This may be due to differing patient characteristics, refinements in risk stratification, therapeutic improvements, or the use of thrombolytics, which may prevent long-term remodeling by maintaining arterial patency. In addition, patients who achieve early coronary reperfusion during AMI frequently exhibit regional myocardial stunning that can persist for weeks.27,30 Patients with minimal LV dysfunction (i.e., LVEF > 40%) are expected to have a low subsequent mortality rate. The TIMI-II36 and GISSI-237 data confirm a similar and comparably low mortality rate in patients with normal LV function (LVEF > 50%; 1.2%), as reported earlier by the MPRG (see Fig. 35-9).10 However, if the LVEF is reduced due to extensive myocardial stunning that later resolves, cardiac risk could be spuriously overestimated. This may partially explain the lower 1-year mortality rate in patients with LV dysfunction in the TIMI-II and GISSI-2 studies compared to the MPRG. Measuring LVEF 1 to 2 months after infarction rather than within the first 2 weeks would reduce the confounding influence of stunning and offer a more reliable estimate of risk. Despite these caveats, the final LVEF remains an important predictor of long-term survival irrespective of initial therapy during STEMI.
Left Ventricular Volumes
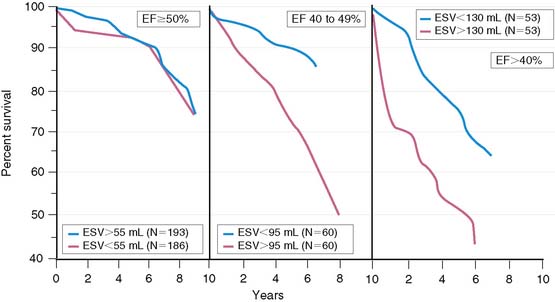
Left ventricular enlargement increases mortality in patients with AMI and particularly when coexisting myocardial dysfunction is present. White and colleagues showed that survival decreased with progressive LV dilation and a decrease in LVEF.13 However, LV dilation influenced survival only in patients with LVEF less than 50% (Fig. 35-14). Likewise, the SAVE investigators demonstrated in 512 patients with LVEF less than 40% that 1-year survivors had a significantly smaller increase in LV dimensions, compared to patients who died.14 LV enlargement is also known to increase mortality in patients with chronic CAD who have a depressed LVEF less than 45%.40
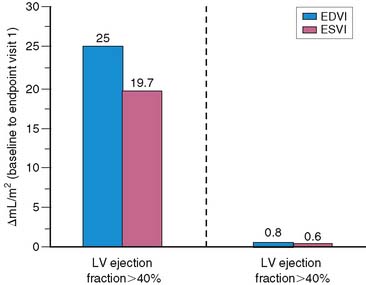
Left ventricular dilation develops early in patients after AMI, presumably as a compensatory mechanism for maintaining stroke volume.41 This is particularly true in patients with anterior infarction, who generally have the greatest degree of initial LV dysfunction and are therefore most likely to develop early infarct zone expansion.41,42 Over the ensuing months, structural and geometric changes occur that entail scar formation and thinning of the infarct zone as well as hypertrophy and dilation of noninfarcted regions.43–45 The initial loss of myocardium, if large enough, leads to progressive LV dilation, increasing wall stress, further LV dysfunction, and ultimately end-stage heart failure. One study in survivors of AMI reported a progressive increase in both LV end-diastolic and end-systolic volumetric indices over a 6-month period, but this occurred almost exclusively in those with an initial LVEF less than 40% (Fig. 35-15).46 Various therapies that limit LV dilation can improve survival in patients with LV dysfunction.14,46 LV volumes can be accurately measured with both gated radionuclide angiography47 and gated SPECT48 techniques.
Myocardial Ischemia
The presence and extent of myocardial ischemia are strong predictors of both fatal and nonfatal cardiac events and improve risk stratification beyond the information gleaned from clinical variables15–18 or the extent of CAD.17,35,49,50 The detection of myocardial ischemia using exercise electrocardiography51–59 has been largely supplanted by more sensitive techniques, including stress echocardiography,60,61 exercise radionuclide angiography,62,63 and most important, stress myocardial perfusion scintigraphy.15–18
RISK STRATIFICATION FOLLOWING ACUTE MYOCARDIAL INFARCTION
Exercise Stress Testing
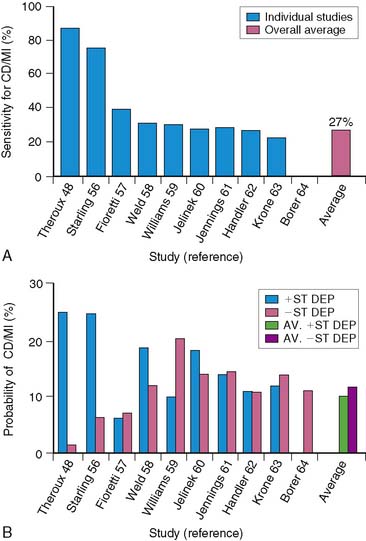
Exercise stress testing has been extensively studied for identifying high- and low-risk survivors of AMI.51–59 Predictors of high risk include a poor exercise effort (<4 METs) and exercise-induced angina, ischemic (>1 mm) ST-segment depression, hypotension, and ventricular arrhythmias. Inability to perform a predischarge exercise test is, in itself, a poor prognostic finding.37,64 In the TIMI-II trial, the mortality rate at 1 year was 7.7% in those who did not perform an exercise test, compared to 1.8% in those who did (P < 0.001).64 In GISSI-2, the mortality rate at 6 months increased from 1.3% to 9.8% based on whether patients could perform an exercise test.37
Electrocardiographic (ECG) ischemia during submaximal exercise predicts subsequent cardiac death.51 In an early study from the Montreal Heart Institute, the 1-year mortality rate among all patients was 9.5%, but death occurred almost exclusively in the 30% of patients with ECG ischemia. Patients without ischemia had only a 2.1% mortality, compared to a 27% mortality in those with ST-segment depression.51
Low-level exercise ECG testing predicts mortality in seemingly low-risk groups after AMI, but it is of limited value in predicting other morbid events. The stress ECG is insensitive for detecting significant CAD,52,65 particularly in patients who perform only submaximal exercise.66 The exercise ECG is much less accurate in risk stratification than myocardial perfusion scintigraphy.16,18,49 Furthermore, an ischemic ECG response during treadmill exercise currently occurs less frequently than previously reported. In the prethrombolytic era, approximately 31% of patients with uncomplicated AMI exhibited ECG ischemia on predischarge exercise testing.15,16,67,68 However, this has decreased to approximately 15% among patients evaluated in the thrombolytic era.35,50,64,69–71 All of these factors limit the ability of the treadmill test to accurately predict which stable patients after AMI are at increased risk for subsequent events (Fig. 35-16).
Gated Radionuclide Angiography
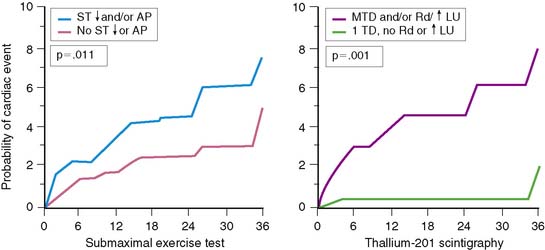
Gated radionuclide angiography allows assessment of LVEF at rest and during dynamic bicycle exercise (see Chapter 11). The resting LVEF identifies patients at high risk for death,6,10–12,35–37 and the presence of exercise-induced ischemia may further improve risk stratification, depending on the population studied and the types of subsequent events considered (Table 35-1).62,63,72–77 Morris and coworkers studied 106 patients, of whom 24 died and an additional 38 had either recurrent AMI, readmission for unstable angina, or refractory angina necessitating coronary revascularization.77 The resting and exercise LVEF both predicted mortality but no other cardiac event. The lack of change in LVEF from resting to exercise identified patients at high risk for developing refractory angina who then underwent coronary revascularization. Abraham and colleagues reported a 58% event rate at 2 years in patients with an exercise LVEF less than 50%, compared to a 17% event rate among those with normal LV function.72 A more than 5% increase in the LVEF during exercise identified a very low-risk group for subsequent cardiac events, particularly if the resting function was greater than 40%.
Table 35-1 Gated Exercise Radionuclide Angiography for Risk Assessment after Acute Myocardial Infarction
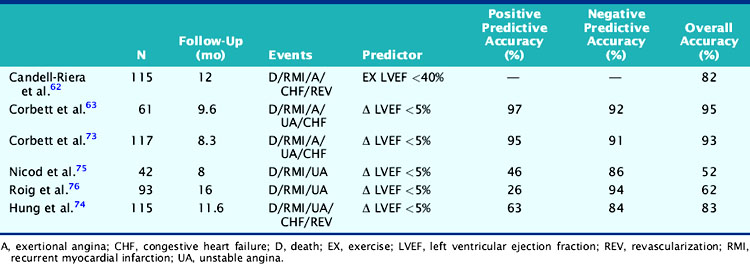
In a study by Hung and colleagues, 115 patients had resting/exercise radionuclide angiography 3 weeks after AMI.74 Twenty-two patients subsequently died (n = 3) or had recurrent infarction (n = 5), readmission for unstable angina (n = 4), congestive heart failure (n = 1), or need for bypass surgery (n = 9), primarily due to refractory angina. The change in LVEF during exercise was a significant predictor of both hard (i.e., death, recurrent infarction) and all cardiac events. Corbett and coworkers showed that 97% of patients who failed to increase their LVEF during exercise returned with a cardiac event by 6 months; however, most of these events were ischemic (i.e., angina or recurrent infarction).63 Conversely, in the series by Nicod and coworkers, where the majority of events were death or recurrent infarction (65%), the exercise LVEF was less discriminating (positive predictive accuracy = 46%).75 Knowing the extent of resting and inducible LV dysfunction appears to identify patients who might best be further evaluated with coronary angiography.
Patients who receive thrombolytic therapy frequently have exercise-induced ischemic LV dysfunction. In the TIMI-II trial, 59% of 2143 patients who had resting and exercise radionuclide angiography prior to hospital discharge had an ischemic response, defined as either a less than 5% increase (48%) or a greater than 5% decrease (11%) in exercise LVEF.36 The 1-year mortality rate in the total TIMI cohort of 3197 patients was 3%, which decreased to 2.2% in the 2567 who underwent gated radionuclide angiography and 1.7% in those who had both resting and exercise radionuclide angiography. Although the resting (see Fig. 35-9), peak exercise (Fig. 35-17), and change in LVEF with exercise all predicted survival, the exercise variables did not improve predictive accuracy over the resting LVEF alone. This result may partially be explained by the exclusion of 1045 patients who could not exercise and had a high mortality rate of 5.8% (see Fig. 35-17). The exercise LVEF variables may also better predict nonfatal ischemic cardiac events, which were not evaluated in this trial. Although important from an historical perspective, exercise gated radionuclide angiography has been largely supplanted by gated SPECT perfusion imaging for risk stratification.
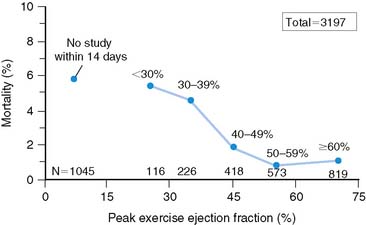
Figure 35-17 Relation of all-cause mortality to peak exercise ejection fraction.
(From Zaret BL, Wackers FJT, Terrin ML, et al, for the TIMI Study Group: Value of radionuclide rest and exercise left ventricular ejection fraction in assessing survival of patients after thrombolytic therapy for acute myocardial infarction: Results of Thrombolysis in Myocardial Infarction (TIMI) Phase II Study, J Am Coll Cardiol 26:73, 1995.)
Exercise Myocardial Perfusion Scintigraphy
Exercise myocardial perfusion scintigraphy can accurately define risk in stable survivors of AMI (Table 35-2).15,16,50,68,78 Patients without scintigraphic ischemia have a very low cardiac event rate (<5%), whereas 40% to 50% of patients with ischemia will develop subsequent cardiac events. Early reports demonstrated increased lung uptake of 201Tl and ischemia in multiple vascular territories as additional high-risk predictors.16 With the advent of quantitative SPECT analysis, the size of the stress-induced perfusion defect, in relation to the presence and quantified extent of scintigraphic ischemia, has added a new dimension to risk stratification.17 Furthermore, with gated SPECT, the important prognostic variables of LVEF and LV volumes can be calculated directly from the perfusion images (see Chapter 12).
Table 35-2 Comparison of Exercise Versus Pharmacologic Coronary Vasodilators for Risk Assessment after Acute Myocardial Infarction
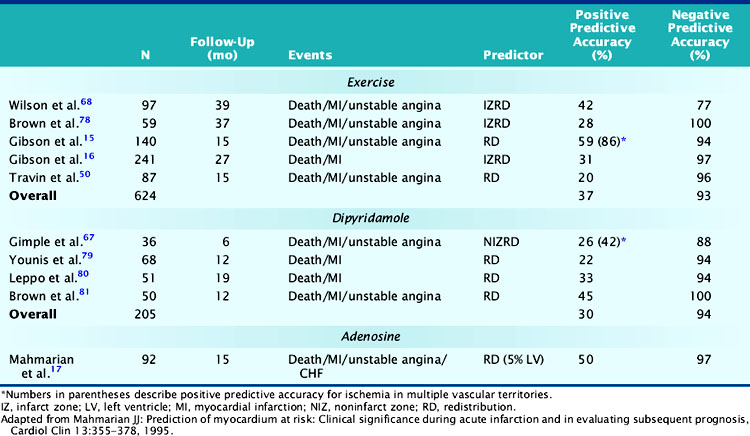
In a landmark study from Gibson and colleagues, 140 seemingly low-risk patients were evaluated with submaximal exercise 201Tl scintigraphy and coronary angiography.16 Over 15 ± 12 months of follow-up, 36% of patients either died (n = 7), had recurrent AMI (n = 9), or had readmission for unstable angina (n = 34). The presence of scintigraphic ischemia, particularly when involving multiple vascular territories, was the most powerful prognosticator. The scintigraphic variables were superior to the treadmill exercise variables in defining high- and low-risk individuals. Fifty-nine percent of patients with evidence of 201Tl redistribution and 86% of those with redistribution in multiple vascular beds (an indicator of multivessel CAD) had a subsequent cardiac event (Fig. 35-18), compared to only 49% of those with ECG ischemia. Moreover, only 6% of patients without scintigraphic ischemia had a cardiac event versus 26% of those with a low-risk exercise test (see Fig. 35-18).
More recently, Travin and colleagues demonstrated the value of exercise 99mTc sestamibi SPECT in stratifying risk after AMI.50 Submaximal exercise SPECT was performed in 134 stable patients within 14 days (mean, 7.5 ± 2 days) of AMI. Ischemic ECG changes were observed in only 23% of patients, whereas 70% had scintigraphic ischemia. Thirty-three patients who had early coronary revascularization were excluded from analysis, and most (79%) of these patients had ischemia by SPECT. Cardiac events occurred in 13 patients over 15 ± 10 months of follow-up. Patients without scintigraphic ischemia had a very low 7% event rate. In patients with ischemia, both the presence and extent of this variable predicted outcome. Overall, 19% of patients with ischemia had a subsequent cardiac event, but this increased from 12% in those with one or two ischemic defects to 38% in patients with more than three ischemic defects. By Cox regression analysis of clinical, exercise treadmill, and scintigraphic variables, only the number of ischemic defects on SPECT predicted outcome.
Pharmacologic Stress Perfusion Scintigraphy
Dipyridamole and adenosine stress are effective and preferable to exercise SPECT as methods for assessing risk in stable patients after AMI.17,67,79–81 Pharmacologic vasodilators maximize heterogeneity in coronary blood flow and can thereby accurately identify the extent of LV hypoperfusion and residual ischemia.82,83 Adenosine induces a similar perfusion defect size as observed with maximal exercise stress.84 Since these tests can be safely performed even within 1 to 2 days after AMI, patients can be rapidly triaged to early coronary angiography or hospital discharge.85–88 The scintigraphic risk variables identified with exercise stress have been confirmed in studies using dipyridamole and adenosine (see Table 35-2).
Dipyridamole SPECT
In the initial series by Leppo and colleagues, dipyridamole 201Tl scintigraphy was performed in 51 patients 1 to 2 weeks after uncomplicated AMI.80 The presence of 201Tl redistribution was the only significant predictor of cardiac death or recurrent AMI. Brown and coworkers safely performed dipyridamole imaging in 50 stable patients very early (mean, 62 ± 121 hours) after hospitalization.81 The only significant predictor of in-hospital and late cardiac events among clinical, scintigraphic, and angiographic variables was the presence of infarct zone 201Tl redistribution. Conversely, none of the patients without redistribution had a subsequent cardiac event over the ensuing year. Other investigators have confirmed the prognostic importance of 201Tl redistribution on dipyridamole imaging after AMI.89,90
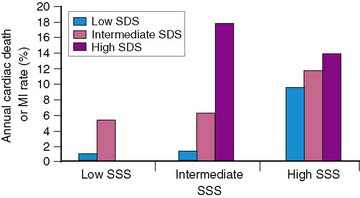
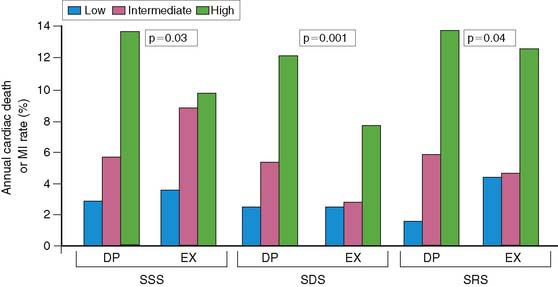
Brown and associates reported the results of a large multicenter trial evaluating dipyridamole SPECT for predicting early and late cardiac events.18 Stable patients postinfarction underwent 3:1 randomization to either early (2 to 4 days) dipyridamole 99mTc sestamibi SPECT followed by exercise SPECT (at 6 to 12 days) (n = 284) or submaximal exercise SPECT alone (n = 309). Twenty-nine patients who had in-hospital cardiac events and 24 who had early coronary revascularization were excluded from long-term follow-up. Following hospital discharge, death or recurrent AMI occurred in 37 patients assigned to dipyridamole testing and in 31 who had submaximal exercise SPECT. A semiquantitative summed stress score (SSS) and summed difference score (SDS) were generated to assess the size of the stress-induced perfusion defect and the extent of scintigraphic ischemia, respectively. Multivariate predictors of in-hospital events among clinical, dipyridamole stress ECG, and scintigraphic variables were only the SSS, SDS, and peak creatine kinase. Multivariate predictors of death or MI following hospital discharge were the dipyridamole SPECT-derived SSS and SDS, as well as anterior infarction location. The extent of scintigraphic ischemia (i.e., SDS) further improved risk stratification—particularly in patients with intermediate-size perfusion defects (Fig. 35-19). Risk stratification was significantly better with dipyridamole than with submaximal exercise SPECT (Fig. 35-20). This study emphasizes that pharmacologic stress testing after infarction is safe and effective at identifying low- and high-risk groups based on the extent of stress-induced hypoperfusion and scintigraphic ischemia. This is the first study to demonstrate improved risk stratification with dipyridamole stress, compared to submaximal treadmill exercise in the same patients.
Adenosine SPECT
Adenosine is a potent direct coronary artery vasodilator that predictably induces maximal hyperemia82,83 and thereby produces a similar perfusion defect extent as observed with maximal exercise stress in patients who have CAD.84 Based on these considerations, its high safety profile,87,88 and exceedingly short half-life,82 this agent is well suited for evaluating patients after AMI for residual myocardial ischemia.
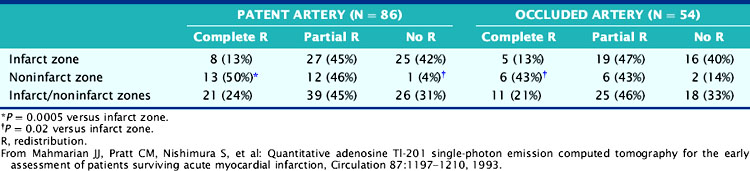
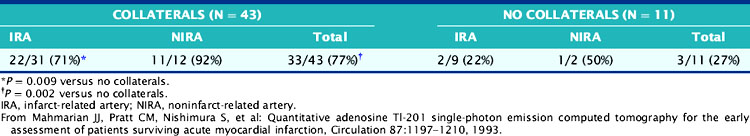
The role of adenosine 201Tl SPECT for detecting residual ischemia and predicting in-hospital cardiac events was first reported in 120 stable survivors of AMI who were imaged early (5 ± 3 days) after infarction.86 The overall sensitivity for detecting significant (>50%) CAD was 87%. Sixty-three percent of patients with double-vessel and 91% of patients with triple-vessel CAD were accurately predicted to have multivessel involvement. Scintigraphic ischemia was common within the infarct zone (59%) but also in noninfarct-zone (63%) territories in patients with multivessel CAD. Neither angiographic patency of the infarct and noninfarct-related arteries (Table 35-3) nor the presence of collaterals (Table 35-4) predicted the presence of scintigraphic ischemia.
Table 35-4 Prevalence of Redistribution Associated with Occluded Infarct and Noninfarct Arteries: Relation to Coronary Collaterals
The adenosine-induced LV perfusion defect size was significantly larger in the 41 patients with in-hospital complications (45 ± 15%) compared to those without such complications (22 ± 15%) (Table 35-5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree