Primary percutaneous coronary intervention (PPCI) for ST-elevation myocardial infarction compares favorably to thrombolysis. In previous studies the benefit has been restricted to the early postinfarction period with no additional risk decrease beyond this period. Long-term outcome after use of third-generation thrombolytics and modern adjunctive pharmaceutics in the 2 treatment arms has not been investigated. This study was conducted to compare 5-year outcome after updated regimens of PPCI or thrombolysis. Patients with ST-elevation myocardial infarction were randomized to enoxaparin and abciximab followed by PPCI (n = 101) or enoxaparin followed by reteplase (n = 104), with prehospital initiation of therapy in 42% of patients. Data on survival and major cardiac events were obtained from Swedish national registries after 5.3 years. PPCI resulted in a better outcome with respect to the composite of death or recurrent myocardial infarction (hazard ratio 0.54, confidence interval 0.31 to 0.95) compared to thrombolysis. This was attributed to a significant decrease in cardiac deaths (hazard ratio 0.16, confidence interval 0.04 to 0.74). The difference evolved continuously over the 5-year follow-up. After adjustment for covariates, a significant benefit remained with respect to cardiac death or recurrent infarction but not for the composite of total survival or recurrent myocardial infarction (p = 0.07). The observed differences were not seen in patients in whom therapy was initiated in the prehospital phase. In conclusion, PPCI in combination with enoxaparin and abciximab compares favorably to thrombolysis in combination with enoxaparin with a risk decrease that stretches beyond the early postinfarction period. Prehospital thrombolysis may, however, match PPCI in long-term outcome.
Primary percutaneous coronary intervention (PPCI) for ST-elevation myocardial infarction has compared favorably to thrombolytic therapy in several previous studies reporting short-term outcome. Previous studies were initiated in a period when first-generation thrombolytics, only in-hospital initiation of therapy, and/or low rates of rescue or emergency angioplasties were used in the thrombolytic therapy group or with no or only low rates of stent implantation or glycoprotein (GP) IIb/IIIa receptor blockers in the PPCI group.
The Swedish Early Decision Reperfusion Strategy (SWEDES) trial compared PPCI to early thrombolysis and aimed to use updated regimens of the 2 strategies based on evidence available at the time the study was initiated (2001) and to preferably prehospital initiation of medical therapy. Although more patients in the PPCI group than in the thrombolysis group had Thrombolysis In Myocardial Infarction grade 3 flow in the infarct-related artery on angiogram within 7 days after inclusion, this did not translate into any statistically significant clinical benefit from PPCI at 30 days. The aim of the present study was to further explore the outcome over >5 years with respect to hard clinical end points in relation to randomization.
Methods
The design, inclusion and exclusion criteria, and methods used in the SWEDES trial have been presented previously. In short, 205 patients with stable circulation and ST-elevation myocardial infarction were randomized to PPCI with abciximab administered before angiography or to thrombolysis at 7 Swedish hospitals, 4 of which had in-house PCI facilities. Patients randomized to PPCI received aspirin and intravenous boluses of enoxaparin (0.75 mg/kg body weight) and abciximab (0.25 mg/kg body weight) at presentation followed by a 12-hour infusion at 10 μg/min. Stent implantation was encouraged in the study protocol. Drug-eluting stents were not available at the time of the study. Clopidogrel was administered as a bolus of 300 mg orally in conjunction with PCI and continued at 75 mg/day for 1 month thereafter. Patients randomized to thrombolysis received aspirin and an intravenous bolus of enoxaparin 30 mg followed by reteplase as a double-bolus injection of 10 + 10 U at a 30-minute interval. Enoxaparin was continued as subcutaneous injections of 1 mg/kg body weight every 12 hours during hospital stay. Patients in this group did not receive clopidogrel unless they underwent a subsequent angioplasty procedure with stenting.
In accordance with the protocol, patients were considered for rescue PCI after thrombolytic treatment if they had ongoing pain and <30% resolution of ST-segment elevation 90 minutes after initiation of thrombolysis or recurrence of chest pain or new ST-segment elevation within 24 hours. Coronary angiography was mandated in all patients before hospital discharge, preferably 5 to 7 days after randomization. The indication for revascularization based on this or subsequent angiography was significant stenosis/stenoses in combination with anginal symptoms or demonstrated ischemia on electrocardiographic monitoring or exercise stress before coronary angiography.
The study was approved by the local ethics committees and the Swedish Medical Products Agency and has been registered at http://www.clinicaltrials.gov (registration number NCT 00806403 ).
The patients were followed prospectively for 1 year according to protocol. Additional approval for an extended follow-up was obtained from the local ethics committee and the epidemiologic center at the Swedish National Board of Health and Welfare. For this follow-up, merged data from the National Patient Registry, the National Cause of Death Registry, the Swedish Coronary Angiography and Angioplasty Registry, and the National Registry of Thoracic Surgery were retrieved in May 2009. At this time, data on survival, cause of death, causes of readmissions, and revascularization procedures were available up to December 31, 2007. Survival confirmation was performed by cross-checking with the Swedish National Population Registry.
The National Cause of Death Registry records all deaths of Swedish residents. Causes of death are based on death certificates that are reported for >99% of all deaths and coded according to the International Classification of Diseases, Tenth Revision (ICD-10). Coding errors with respect to ICD-10 chapter and block levels have been estimated to <2%. Accuracy of ischemic heart disease as cause of death has previously been estimated to approximately 95%.
Cause of death was categorized as cardiac, which included all manifestations of ischemic heart disease and complications after acute myocardial infarctions (ICD-10 codes I20.0 to I25.9), heart failure (I50.0 to I50.9), ventricular arrhythmias (I47.2 and I49.0), cardiac arrest including sudden cardiac death (I46.0 to I46.9), and other sudden death (R96). Other causes were categorized as noncardiac. Outcome measurements were all-cause death, cardiac death, and their combinations with recurrent myocardial infarction, whichever occurred first.
All comparisons between treatment groups are based on the intention-to-treat principle. Between-group comparisons of categorical variables were made with Fisher’s exact test. Kaplan-Meier product–limit method was used to construct survival curves and groups were compared using log-rank test. When constructing curves for cardiac death and its composite, patients who died from noncardiac causes were censored. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were obtained from univariable Cox proportional hazard regression analyses. Baseline characteristics that are known to be of importance for long-term outcome (age, previous myocardial infarction, heart failure, diabetes, smoking, gender) and site of initiation of treatment were entered in adjusted Cox regression models. Follow-up time was computed with the reverse Kaplan-Meier method where the outcomes “death” and “censored” were exchanged and the median time to censoring was used. Time to treatment and follow-up time were reported as median with first and third quartiles, unless stated otherwise. A 2-tailed p value <0.05 was considered statistically significant. Statistics 17.0 (SPSS, Inc. Chicago, Illinois) was used for statistical analyses.
Results
From November 2001 to May 2003, 101 patients were randomized to PPCI and 104 patients to thrombolysis. Forty-three percent of patients in the PPCI group received abciximab and enoxaparin before arrival at the hospital and 41% of patients in the thrombolysis group received prehospital thrombolysis. Median time from onset of symptoms to administration of enoxaparin/reteplase in the thrombolysis group was 114 minutes (83 to 197) and from onset of symptoms to administration of enoxaparin/abciximab in the PPCI group was 120 minutes (85 to 210). Ninety-six percent of patients were admitted directly to an angioplasty center. In patients who underwent PPCI, stent implantation rate was 95%. In lytic-treated patients, 22% underwent rescue angioplasty within a median of 2.2 hours (minimum 0.5, maximum 8.8) after start of lytic therapy, and a further 24% underwent revascularization before hospital discharge. Median duration of follow-up was 5.3 years (5.0 to 5.8).
Patients were well matched in baseline characteristics and medication at discharge and at 1 year, except for clopidogrel use at hospital discharge ( Table 1 ).
| Variable | PPCI | Thrombolysis |
|---|---|---|
| (n = 101) | (n = 104) | |
| Age (years), mean ± SD | 65.3 ± 10.9 | 64.3 ± 12.4 |
| Men/women | 74/27 | 78/26 |
| Previous angina pectoris | 19 | 16 |
| Previous myocardial infarction | 13 | 16 |
| History of heart failure | 2 | 3 |
| History of hypertension | 32 | 29 |
| Diabetes mellitus | 14 | 11 |
| Previous coronary artery bypass grafting | 4 | 2 |
| Previous percutaneous coronary intervention | 6 | 7 |
| Current smoker (5/5) ⁎ | 29 | 29 |
| Prehospital initiation of treatment | 43 | 42 |
| Anterior wall myocardial infarction | 42 | 40 |
| Baseline maximum ST elevation (mm), mean ± SD (20/19) ⁎ | 4.2 ± 2.5 | 4.6 ± 2.4 |
| Medication at discharge | ||
| Number of patients | 98 | 100 |
| Aspirin | 95 | 93 |
| Clopidogrel | 89 | 51 |
| β-Blocker | 93 | 96 |
| Angiotensin converting enzyme inhibitor/angiotensin II receptor blocker | 57 | 52 |
| Statin | 83 | 83 |
| Medication at 1-year follow-up | ||
| Number of patients | 93 | 89 |
| Aspirin | 86 | 82 |
| Clopidogrel | 5 | 6 |
| β-Blocker | 80 | 81 |
| Angiotensin converting enzyme inhibitor/angiotensin II receptor blocker | 58 | 44 |
| Statin | 79 | 75 |
⁎ Number of patients for whom information was missing in the 2 groups, respectively.
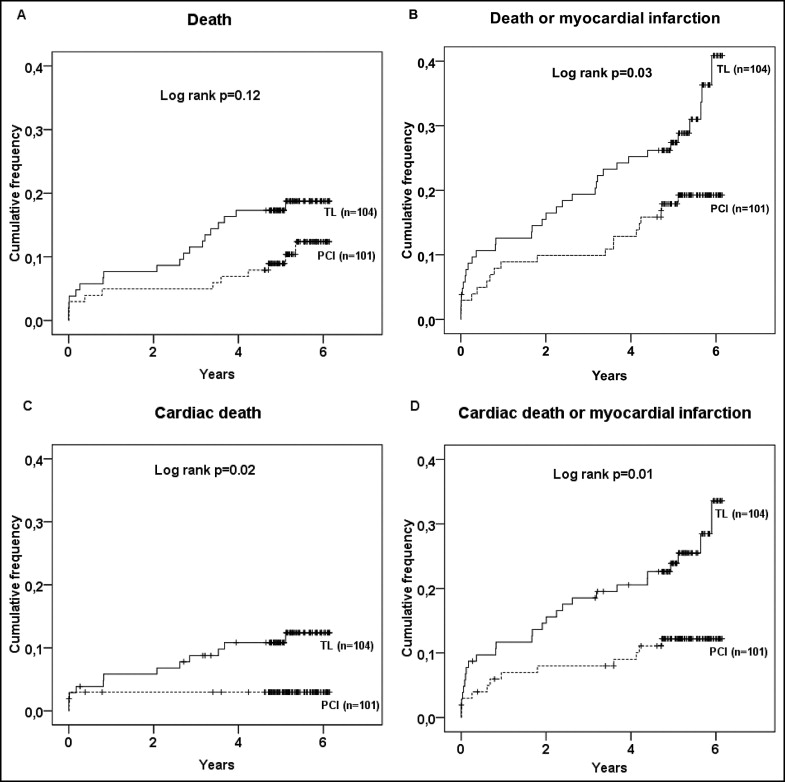
Information on clinical events during the 5.3-year follow-up was available in all patients ( Tables 2 and 3 ). There was a trend toward a lower total mortality rate in the PPCI group compared to the thrombolysis group, a difference that was attributed to a significant decrease in cardiac deaths. Of the 3 cardiac deaths in the PPCI group, which occurred during primary hospitalization, 1 was due to a proximal aortic dissection causing a myocardial infarction. Incidence of the combined end point of death or recurrent myocardial infarction was significantly lower in the PPCI group. Furthermore, revascularization procedures after the initial reperfusion treatment were more common in the thrombolysis group than in the PPCI group. During the 5.3-year follow-up period, crude rates of hospital readmission due to angina and heart failure were 28% and 7% in the PPCI group versus 38% and 4% in the thrombolysis group (p = NS for the 2 comparisons).
| Cause of Death (ICD-10 code) | PPCI | Thrombolysis |
|---|---|---|
| (n = 101) | (n = 104) | |
| Cardiac | ||
| Myocardial infarction (I21.9) | 3 ⁎ | 11 |
| Chronic ischemic cardiac disease (I25.9) | 0 | 1 |
| Noncardiac | ||
| Cerebrovascular disease (I61–I64) | 4 | 1 |
| Malignancy (C18–C80) | 3 | 4 |
| Dementia (F039) | 1 | 0 |
| Other nonspecified (R999) | 0 | 2 |
⁎ Including 1 case of aortic dissection causing the index myocardial infarction.
| Cumulative Number of Patients With Clinical Events | PPCI | Thrombolysis | p Value |
|---|---|---|---|
| (n = 101) | (n = 104) | ||
| Myocardial infarction | |||
| 30 days | 0 | 2 | 0.50 |
| 1 year | 4 | 7 | 0.54 |
| 5 years | 9 | 18 | 0.10 |
| Death | |||
| 30 days | 3 | 4 | 1.00 |
| 1 year | 5 | 8 | 0.57 |
| 5 years | 11 | 19 | 0.17 |
| Cardiac death | |||
| 30 days | 3 | 3 | 1.00 |
| 1 year | 3 | 6 | 0.50 |
| 5 years | 3 | 12 | 0.03 |
| Death or nonfatal myocardial infarction | |||
| 30 days | 3 | 6 | 0.50 |
| 1 year | 9 | 14 | 0.38 |
| 5 years | 19 | 33 | 0.04 |
| Stroke | |||
| 30 days | 0 | 3 | 0.25 |
| 1 year | 0 | 4 | 0.12 |
| 5 years | 6 | 8 | 0.78 |
| Any revascularization excluding primary percutaneous coronary intervention | |||
| 30 days | 7 | 59 | <0.001 |
| 1 year | 15 | 72 | <0.001 |
| 5 years | 22 | 79 | <0.001 |
Kaplan-Meier curves for the different end points are shown in Figure 1 . Patients randomized to PPCI had a better outcome with respect to the combination of all-cause death or recurrent myocardial infarction (HR 0.54, CI 0.31 to 0.95, p = 0.03), cardiac death alone (HR 0.16, CI 0.04 to 0.74, p = 0.02), and its composite with recurrent myocardial infarction (HR 0.44, CI 0.22 to 0.87, p = 0.01). There was no significant difference in all-cause death, although there was a trend toward a benefit from PPCI (HR 0.56, CI 0.27 to 1.18, p = 0.12). There was a continuing separation of event curves beyond the early postinfarction period.