The risk of stroke in patients hospitalized with an acute coronary syndrome (ACS) ranges from <1% to ≥2.5%. The aim of this study was to develop a simple predictive tool for bedside risk estimation of in-hospital ischemic stroke in patients with ACS to help guide clinicians in the acute management of these high-risk patients. Data were obtained from 63,118 patients enrolled from April 1999 to December 2007 in the Global Registry of Acute Coronary Events (GRACE), a multinational registry involving 126 hospitals in 14 countries. A regression model was developed to predict the occurrence of in-hospital ischemic stroke in patients hospitalized with an ACS. The main study outcome was the development of ischemic stroke during the index hospitalization for an ACS. Eight risk factors for stroke were identified: older age, atrial fibrillation on index electrocardiogram, positive initial cardiac biomarkers, presenting systolic blood pressure ≥160 mm Hg, ST-segment change on index electrocardiogram, no history of smoking, higher Killip class, and lower body weight (c-statistic 0.7). The addition of coronary artery bypass graft surgery and percutaneous coronary intervention into the model increased the prediction of stroke risk. In conclusion, the GRACE stroke risk score is a simple tool for predicting in-hospital ischemic stroke risk in patients admitted for the entire spectrum of ACS, which is widely applicable to patients in various hospital settings and will assist in the management of high-risk patients with ACS.
Although stroke is a relatively uncommon complication in patients hospitalized with an acute coronary syndrome (ACS), it has a considerable impact on short-term and long-term clinical outcomes. Previous studies have shown that the incidence of stroke after an ACS ranges from <1% to ≥2.5%. In-hospital stroke has been found to be an independent predictor of hospital mortality, and death rates remain high in patients discharged from hospital after developing a stroke in the setting of an ACS. Furthermore, ≥40% of patients who survive a stroke are left with moderate to severe neurologic deficits, resulting in significant morbidity and impaired quality of life. Most current stroke data are derived from mega-trials performed in patients treated with thrombolytics, a population in whom there is a shift toward more hemorrhagic stroke and resultant mortality. Therefore, these data may be misleading given the increasing use of percutaneous coronary interventions (PCIs) and newer antithrombotic and antiplatelet drugs in patients hospitalized with ACS. Furthermore, ischemic stroke has always been a more common diagnosis, comprising approximately 60% to 90% of acute strokes, and modification of the risk factors contributing to ischemic stroke has a wider potential scope for effect. A clinical prediction model estimating the risk of ischemic stroke in patients hospitalized with ACS would help to identify higher-risk patients and assist physicians in critical management decisions. Previous analyses of randomized controlled trials and observational studies have identified a limited number of demographic and clinical risk factors associated with an increased likelihood of stroke in patients with ACS. However, these data were derived from old and highly selected populations and therefore may have limited current generalizability. We used data from the Global Registry of Acute Coronary Events (GRACE) study to develop a risk-prediction model for the development of in-hospital ischemic stroke in patients hospitalized with ACS.
Methods
Full details of the GRACE methods have been published. GRACE is a multinational cooperative effort involving 126 hospitals in 14 countries designed to reflect an unbiased representative population of patients with ACS. Patients eligible to participate in the registry are ≥18 years old, admitted to participating hospitals with a presumptive diagnosis of ACS, and a clinical history of ACS accompanied by electrocardiographic changes consistent with ACS, serial increases in biochemical markers of cardiac necrosis, and/or documented coronary artery disease. When required, study investigators received approval from their local hospital ethics or institutional review boards for the conduct of this study.
To ensure enrollment of an unbiased population, the first 10 to 20 consecutive eligible patients were recruited from each site per month. Data were collected by trained coordinators using a standardized case-report form. Demographic characteristics, medical history, presenting symptoms, biochemical and electrocardiographic findings, treatment practices, and hospital outcome data were collected. All cases were assigned to 1 of the following categories: ST-segment-elevation myocardial infarction, non–ST-segment-elevation myocardial infarction, or unstable angina using standardized definitions and criteria. Standardized definitions were used for selected hospital complications and outcomes ( http://www.outcomes-umassmed.org/grace/ ). Stroke (hemorrhagic, nonhemorrhagic, other) was defined according to the occurrence of typical neurologic signs and symptoms as diagnosed by the patient’s attending physician. Use of computed tomography and/or magnetic resonance imaging was documented.
The primary outcome was the development of in-hospital ischemic stroke within 14 days of admission for ACS. A 14-day cutoff was used to accommodate different lengths of hospital stay and because most in-hospital strokes would have developed during this period. We used the Kaplan–Meier method to estimate unadjusted 14-day stroke risk. Univariate comparisons for patient characteristics of patients with and without stroke were made using Fisher’s exact test (for discrete variables) or Wilcoxon rank-sum test (for continuous variables). We assessed potential nonlinear associations between continuous variables and stroke using the fractional polynomials method, but none were found.
Cox regression was used to develop a prediction model for ischemic stroke. We considered all factors whose univariate association with stroke showed a p value ≤0.25, retaining those whose adjusted association showed a p value ≤0.05. We evaluated the proportional hazards assumption for this model by testing the interaction between time to event and each model factor.
Because 19% of patients had missing information on ≥1 final model covariate, we used multiple imputation to estimate a second model for all possible patients: (1) a multiple imputation procedure (SAS PROC MI; SAS 9.1, SAS Institute, Cary, North Carolina) created 5 datasets with different sets of values for the missing data using a Markov chain Monte Carlo method; (2) separate Cox multiple regression models were fit to each of the 5 datasets; and (3) the 5 sets of regression results were pooled to estimate a single set of final model results (SAS PROC MIANALYZE).
A “sensitivity analysis” was performed using available data on the 30% of patients with stroke who were classified as “other” stroke or missing stroke types and were unable to be accounted for in our study. When we made an assumption that the patients who did not receive fibrinolytics had ischemic strokes and included these patients into our ischemic stroke model, there was no significant change in our stroke model data.
To assess how well our model predicts stroke, we compared model-predicted with observed rates. We ranked patient scores from low to high and then divided patients into 10 roughly equal deciles of stroke risk. We computed model-predicted rates for each patient by obtaining the Cox model 14-day baseline nonstroke rate (S 0(14) ), and the product of each patient’s individual covariate values and the model estimates for those covariates (Xβ). Each patient’s estimated 14-day stroke rate is 1 − S 0(14) Xβ-hat . From this we computed mean 14-day predicted stroke rates for each risk score decile. We used the Kaplan–Meier method to estimate observed 14-day rates by decile. We then plotted mean versus observed rates by median decile risk score.
This process was followed for the model incorporating coronary revascularization methods, except that PCI and coronary artery bypass grafting (CABG) were modeled as time-varying covariates (TVCs). The TVC model does not provide predicted stroke risk. To obtain predicted stroke rates, we reconstituted our data using the counting process setup, where each patient contributes a record for each interval in which covariates and outcome are stable. Cox regression on the counting process data provides a predicted stroke probability by day t for each stable patient interval (day t is the interval end point). The predicted stroke rate for the last patient interval is the basis for the final adjusted day-14 stroke risk (1 − S 0(14) Xβ-hat , where X is the set of covariate values for the last patient interval). It should be noted that model estimates for the usual TVC model and the counting process data TVC model were identical. SAS 9.1 was used for all statistical analyses.
Results
In total, 70,359 patients were enrolled in GRACE from April 1999 to December 2007. In the 65,127 patients confirmed to have ACS, there were 468 strokes (0.7%, 240 ischemic [51%], 87 hemorrhagic [19%]), and 141 were of another type or the type of stroke was not recorded (30%). After excluding 1,781 patients with missing data on ischemic stroke and PCI/CABG and 228 patients with hemorrhagic or unknown stroke type, analyses were performed in 63,118 patients. Of these, 217 developed an ischemic stroke during hospital admission, and the 14-day stroke rate using the Kaplan–Meier method was 0.65%. Use of computed tomography/magnetic resonance imaging to confirm the diagnosis of stroke was documented in >2/3 of stroke cases. Approximately 1/4 of strokes occurred at hospital presentation or during the first day of admission and 2/3 of all strokes occurred within the first 4 days after admission. Median hospital length of stay was 5 days.
Compared to patients who did not develop an acute stroke during their hospital admission, patients who developed an ischemic stroke were significantly older, more likely to be women, have a lower body mass index (BMI), were more likely to have a history of transient ischemic attack/stroke, and hypertension, and were not previous smokers ( Table 1 ). On initial presentation, patients with stroke were more likely to have atrial fibrillation or atrial flutter on index electrocardiogram, higher Killip class, ST-segment deviation, positive cardiac biomarkers, renal insufficiency, systolic blood pressure ≥160 mm Hg, cardiac arrest ( Table 2 ), and a higher GRACE mortality risk score ( Table 1 ). There was greater use of aspirin, low-molecular-weight heparin, and statins in patients without stroke ( Table 2 ).
| Variable | No Stroke | Stroke | p Value |
|---|---|---|---|
| (n = 62,901) | (n = 217) | ||
| Age (years) | 66 (56–76) | 73 (64–80) | <0.001 |
| Women | 20,418 (33%) | 100 (46%) | <0.001 |
| Systolic blood pressure (mm Hg) | 140 (120–160) | 140 (117–162) | 0.71 |
| Diastolic blood pressure (mm Hg) | 80 (70–90) | 80 (63–90) | 0.27 |
| Heart rate (beats/min) | 76 (65–90) | 80 (65–95) | 0.08 |
| Weight (kg) | 77 (68–88) | 70 (63–80) | <0.001 |
| Body mass index (kg/m 2 ) | 27 (24–30) | 26 (24–28) | <0.001 |
| GRACE risk score | 129 (105–155) | 154 (129–179) | <0.001 |
| Medical history | |||
| Angiographic coronary artery disease | 19,100 (31%) | 48 (22%) | 0.005 |
| Atrial fibrillation | 4,788 (7.7%) | 23 (11%) | 0.09 |
| Major bleeding | 745 (1.2%) | 3 (1.4%) | 0.75 |
| Congestive heart failure | 6,402 (10%) | 23 (11%) | 0.83 |
| Diabetes mellitus | 15,724 (25%) | 59 (27%) | 0.43 |
| Hyperlipidemia | 30,154 (48%) | 87 (41%) | 0.02 |
| Hypertension | 38,719 (62%) | 154 (71%) | 0.01 |
| Myocardial infarction | 18,922 (30%) | 52 (24%) | 0.05 |
| Peripheral arterial disease | 5,864 (9.4%) | 24 (11%) | 0.41 |
| Renal insufficiency | 4,783 (7.6%) | 20 (9.3%) | 0.37 |
| Any smoking history | 36,049 (58%) | 93 (43%) | <0.001 |
| Former smoker only | 17,833 (29%) | 42 (20%) | 0.003 |
| Current smoker only | 16,938 (28%) | 47 (22%) | 0.09 |
| Transient ischemic attack/stroke | 5,188 (8.3%) | 28 (13%) | 0.02 |
| Unstable angina | 32,763 (52%) | 106 (49%) | 0.38 |
| Coronary artery bypass grafting | 7,762 (12%) | 24 (11%) | 0.61 |
| Previous percutaneous coronary intervention | 10,953 (18%) | 24 (11%) | 0.01 |
| Variable | No Stroke | Stroke | p Value |
|---|---|---|---|
| (n = 62,901) | (n = 217) | ||
| Atrial fibrillation/flutter on index electrocardiogram | 3,486 (5.5%) | 26 (12%) | <0.001 |
| Killip class at presentation | <0.001 | ||
| I | 51,200 (83%) | 150 (70%) | |
| II | 7,347 (12%) | 45 (21%) | |
| III | 2,342 (3.8%) | 13 (6.0%) | |
| IV | 638 (1.0%) | 7 (3.3%) | |
| II to IV | 10,327 (17%) | 65 (30%) | |
| ST-segment deviation on presentation | 34,367 (55%) | 143 (66%) | 0.001 |
| Cardiac arrest on presentation | 1,242 (2.0%) | 9 (4.2%) | 0.02 |
| Positive initial cardiac biomarkers | 28,534 (47%) | 128 (62%) | <0.001 |
| Glomerular filtration rate | <0.001 | ||
| ≥60 (ml/min/1.73 m 2 ) | 40,041 (68%) | 98 (52%) | |
| 30–59 (ml/min/1.73 m 2 ) | 15,885 (27%) | 76 (40%) | |
| <30 (ml/min/1.73 m 2 ) | 2,952 (5%) | 16 (8%) | |
| Systolic blood pressure ≥160 mm Hg | 16,548 (27%) | 72 (35%) | 0.02 |
| Cardiac medications | |||
| Aspirin ⁎ | 59,262 (94%) | 195 (90%) | 0.02 |
| Glycoprotein IIb/IIIa inhibitor † | 10,469 (20%) | 36 (20%) | 0.93 |
| Low-molecular-weight heparin ⁎ | 30,346 (49%) | 76 (37%) | <0.001 |
| Unfractionated heparin † | 25,515 (41%) | 99 (47%) | 0.12 |
| Statin ⁎ | 34,338 (55%) | 86 (41%) | <0.001 |
| Thienopyridine ‡ | 25,195 (41%) | 75 (36%) | 0.16 |
| Thrombolytic therapy § | 7,089 (12%) | 28 (13%) | 0.45 |
| Thrombolytic therapy (in hospital, any time) | 8,357 (13%) | 31 (14%) | 0.62 |
| Warfarin (long term) | 2,749 (4.5%) | 9 (4.4%) | 0.99 |
| Cardiac procedures | |||
| Coronary artery bypass grafting at/before ischemic stroke, censored at 14 days | 2,664 (4.2%) | 23 (11%) | <0.001 |
| Percutaneous coronary intervention at/before ischemic stroke, censored at 14 days | 23,642 (38%) | 60 (28%) | <0.001 |
| Length of hospital stay (days) | 5 (3–9) | 12 (7–17) | <0.001 |
| Discharge diagnosis | <0.001 | ||
| ST-segment elevation myocardial infarction | 22,884 (36%) | 109 (50%) | |
| Non–ST-segment elevation myocardial infarction | 20,634 (33%) | 75 (35%) | |
| Unstable angina | 19,383 (31%) | 33 (15%) |
⁎ Long term, before hospitalization, or in first 24 hours.
† Before hospitalization or in first 24 hours.
‡ Long term or in first 24 hours.
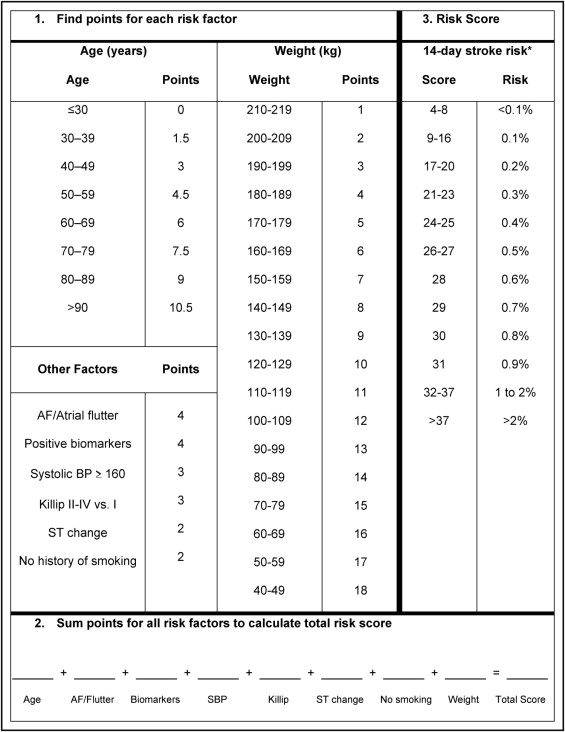
Multivariable analysis found 8 baseline and presenting clinical characteristics that were independently associated with occurrence of ischemic stroke in the 51,034 patients with 164 strokes who had complete information on all 8 characteristics ( Table 3 ). Missing data were imputed to create the final model, which was based on 63,118 observations with 217 ischemic strokes ( Table 4 ). Age was an important risk predictor and there was a roughly linear relation between advancing age and risk of ischemic stroke, with a 20% increased hazard per decade. Other independent predictors included atrial fibrillation or atrial flutter on the patient’s index electrocardiogram, positive initial biomarkers, presenting systolic blood pressure ≥160 mm Hg, presence of ST-segment change on electrocardiogram, no history of smoking, higher Killip class, and lower body weight.
| Risk Factor | HR | 95% CI | p Value | Chi-Square |
|---|---|---|---|---|
| Atrial fibrillation/flutter on index electrocardiogram | 2.22 | 1.42–3.47 | 0.0005 | 12 |
| Systolic blood pressure ≥160 mm Hg | 1.67 | 1.21–2.29 | 0.002 | 9.9 |
| Positive initial cardiac biomarkers | 1.59 | 1.16–2.19 | 0.004 | 8.1 |
| No previous/current smoking | 1.52 | 1.09–2.11 | 0.01 | 6.2 |
| ST-segment change | 1.50 | 1.07–2.09 | 0.02 | 5.6 |
| Killip class II to IV | 1.39 | 0.98–1.97 | 0.07 | 3.4 |
| Per 10-kg decrease in weight | 1.20 | 1.03–1.34 | 0.001 | 12 |
| Per 10-year increase in age | 1.10 | 0.95–1.26 | 0.21 | 1.6 |
⁎ Based on patients (n = 51,034) with complete covariate data and 164 ischemic strokes by day 14 (0.65% stroke risk by Kaplan–Meier method).
| Risk Factor | HR | 95% CI | p Value | Risk Score Points |
|---|---|---|---|---|
| Atrial fibrillation/flutter on index electrocardiogram | 1.64 | 1.07–2.49 | 0.02 | 4 |
| Positive initial cardiac biomarkers | 1.57 | 1.19–2.09 | 0.002 | 4 |
| Systolic blood pressure ≥160 mm Hg | 1.45 | 1.08–1.94 | 0.01 | 3 |
| Killip class II to IV | 1.39 | 1.03–1.89 | 0.03 | 3 |
| No previous/current smoking | 1.36 | 1.02–1.80 | 0.04 | 2 |
| ST-segment change | 1.33 | 1.00–1.77 | 0.05 | 2 |
| Per 10-year increase in age | 1.20 | 1.06–1.36 | 0.004 | 1.5/10 years † |
| Per 10-kg decrease in weight | 1.13 | 1.03–1.24 | 0.01 | 1/10 kg ‡ |
⁎ Based on 63,118 observations with imputation of missing data and 217 ischemic strokes by day 14 (0.65% stroke risk by Kaplan–Meier method).
† Additional 1.5 points per extra 10 years after 30 years of age.
A risk-prediction model for the primary end point of ischemic stroke within 14 days of hospitalization was developed by converting final model estimates to risk points. Weight had the smallest estimate (0.125 on natural logarithmic hazard ratio [HR] scale; Table 4 ). Each 10-kg decrease in weight, starting with the heaviest group (210 to 219 kg), was therefore assigned 1 additional point. The natural logarithmic HR for atrial fibrillation/flutter, 0.493, is about 4 times as large as 0.125, so it was assigned 4 points. Remaining risk factors were assigned points based on their natural logarithmic HRs in relation to 0.125. Points for each of the 8 patient factors were summed to obtain a total patient risk score ( Figure 1 ).