Right-Sided Pulmonary Resections
Larry R. Kaiser
GENERAL INTRODUCTION TO PULMONARY RESECTIONS
Pulmonary resection is the operation that defines the thoracic surgeon. The specialty of thoracic surgery is relatively new dating back to less than 50 years but really being defined during the past 30 years. Because of problems relating to positive pressure ventilation in the patient with an open chest, the development of anatomic pulmonary resection moved slowly. The initial procedure performed for a carcinoma of the lung was a pneumonectomy carried out by mass ligation of the pulmonary hilum with subsequent suturing of individual hilar structures. During the first four decades of the 20th century carcinoma of the lung was an uncommon disease and most pulmonary resections were performed for inflammatory conditions or tuberculosis. Most lung cancers were treated by total removal of the lung when they were deemed operable and this clearly was the operation thought to be required. Lesser resections were reserved for benign disease, mostly infectious problems. It took a number of years before surgeons recognized that an anatomic resection of a lobe, though a more difficult operation, provided an acceptable alternative for the treatment of lung cancer, not unlike the recognition that a more conservative resection than a radical mastectomy could be done for breast cancer with comparable survival rates.
Recognizing that surgical excision is the optimal treatment for otherwise operable lung cancer, it is important that the appropriate procedure be performed. Lobectomy remains the definitive resection since it is an anatomic resection that assures removal of the regional lymph nodes that course along the lobar bronchus and thus provides the best staging information and local control. Doing less than a lobectomy must be considered a compromise though often it is tempting to consider a nonanatomic wedge excision for small primary tumors. Not only does a wedge excision not include the lobar bronchus, precluding evaluation of lobar lymph nodes, but usually also provides only a minimal parenchymal margin and thus is accompanied by a significant incidence of local recurrence. The Lung Cancer Study Group (LCSG) addressed the question of lobectomy versus limited resection for T1N0 lesions (tumor <3 cm, negative lymph nodes) in a prospective randomized trial. The initial analysis of the data demonstrated an increased incidence of local recurrence in the limited resection group (>30% incidence) but failed to demonstrate a decrease in survival. The final analysis, however, revealed superior survival for patients in the lobectomy group. A number of other studies have looked retrospectively at patients undergoing limited resection, including anatomic segmental resection, and have demonstrated long-term survivors but the LCSG study stands as the only large randomized trial. Limited resection is associated with lower morbidity and decreased hospital stays but the best evidence supports a higher incidence of loco regional recurrence when compared to lobectomy. That said, limited resection may be comparable to lobectomy in the elderly (>70 year old) and in those with small peripheral tumors.
There are patients in whom lobectomy is not feasible and a lesser resection offers the best alternative, though admittedly a compromise. Patients in this category are those with borderline pulmonary function or those who have had previous pulmonary resections. Whenever possible the lesser resection should be an anatomic segmental resection, which by definition involves taking the appropriate segmental artery and vein as well as the segmental bronchus with its accompanying lymph nodes. Wedge resection, a nonanatomic form of resection where the bronchovascular structures are not isolated and taken separately along with regional lymph nodes, is another alternative though not ideal for patients with primary lung cancers. With the advent of videothoracoscopic techniques and the simplicity of wedge resection via this approach for a time there was renewed interest in utilizing this technique for T1N0 lung cancers. The American College of Surgeons Oncology Group (ACOSOG) has an ongoing study looking at limited resection combined with local implantation of radioactive seeds to assess whether there is a decreased incidence of local recurrence and whether this translates into a survival benefit. Preliminary results at 60 and 90 days show no increase in morbidity with the addition of brachytherapy when compared with sublobar resection alone. As it currently stands based on the LCSG data, wedge excision mostly should be avoided and patients who are found to have a primary lung cancer should be offered the best possible procedure which is, to the best of present knowledge, a lobectomy. Wedge resection, at best, is a compromise and patients who otherwise can tolerate an anatomic resection are not well served by having a lesser procedure, at least based on the best evidence to date. This has become even more important as we are identifying more early stage, small lung cancers picked up in patients who present themselves for screening spiral computed tomography (CT) scans. As noted above sublobar resection of small peripheral tumors, especially some of those identified on screening spiral CT scans, may have a survival comparable to lobectomy.
Prior to operation a decision must be made regarding which, if any, other studies should be carried out. The type and extent of the staging evaluation depends on a number of clinical factors. At a minimum patients should have a recent chest radiograph and CT scan of the chest. Most, if not all, should have a recent set of pulmonary function studies including diffusion capacity. Positron emission tomography (PET) scanning has become standard for the evaluation of a solitary pulmonary nodule and there is convincing evidence of its usefulness in staging the mediastinum as well. The fluorinated glucose used for the PET scan is trapped preferentially in malignant cells as opposed to normal cells and thus shows up as hot. Numerous studies have looked at the sensitivity and
specificity of PET for both pulmonary nodules and mediastinal lymph nodes. A nodule that shows up positive on PET has a high (approaching 90%) likelihood of being malignant. A negative PET scan does not rule out malignancy as bronchioloalveolar carcinoma and carcinoid tumors do not readily take up the fluorinated glucose and thus have a significantly higher incidence of false-negative results. An elevated blood glucose level also could be the source of a false-negative scan. A solitary nodule that is negative on PET needs to be followed with serial CT scans and resected if growth is detected. In assessing mediastinal lymph nodes, PET has a specificity of greater than 90% but the sensitivity is somewhat lower and approximates to 80%. A CT scan without mediastinal lymph nodes greater than 1.5 cm in size and a negative PET scan obviates the need for further mediastinal staging while a PET-positive lymph node mandates the need for invasive mediastinal staging with either mediastinoscopy or endoscopic bronchial ultrasound (EBUS)-guided needle aspiration.
specificity of PET for both pulmonary nodules and mediastinal lymph nodes. A nodule that shows up positive on PET has a high (approaching 90%) likelihood of being malignant. A negative PET scan does not rule out malignancy as bronchioloalveolar carcinoma and carcinoid tumors do not readily take up the fluorinated glucose and thus have a significantly higher incidence of false-negative results. An elevated blood glucose level also could be the source of a false-negative scan. A solitary nodule that is negative on PET needs to be followed with serial CT scans and resected if growth is detected. In assessing mediastinal lymph nodes, PET has a specificity of greater than 90% but the sensitivity is somewhat lower and approximates to 80%. A CT scan without mediastinal lymph nodes greater than 1.5 cm in size and a negative PET scan obviates the need for further mediastinal staging while a PET-positive lymph node mandates the need for invasive mediastinal staging with either mediastinoscopy or endoscopic bronchial ultrasound (EBUS)-guided needle aspiration.
The decision to search for disseminated disease is a difficult one and precise criteria to define if and when it should be done remain controversial. Our bias is to err on the side of performing a complete extent of disease evaluation if there is any reason at all to do so. Reasons would include any organ-specific or nonspecific signs or symptoms. Nonspecific signs include weight loss, easy fatigability, or anemia while organ-specific symptoms include bone pain, elevated liver enzymes, or localized neurologic findings. If any of these findings is present a complete staging evaluation should be obtained, not just the study pointed to by the organ-specific complaint. A PET scan obviates the need for a separate radionuclide bone scan but an MRI (or less likely) a CT scan of the brain is required for a complete extent of disease evaluation. Any patient with a past history of malignancy should have a search for distant disease as should the patient who is at a higher risk for operation, such as an individual with multiple medical problems or borderline pulmonary function. Also, any patient with locally advanced disease in whom the indications for operation are being extended (i.e., N2 disease, T4 disease) or the nonsmoker with a lung mass should have distant disease ruled out. The aim is to avoid operating on a patient who proceeds to manifest disseminated disease within 1 year of operation, a finding which ideally should have been identified preoperatively.
An important aspect of the preoperative evaluation of a patient with lung cancer is the assessment of pulmonary function. Not all patients undergoing thoracotomy require pulmonary function testing but the majority of patients with lung cancer also have some element of underlying lung disease as a result of the same risk factor that is associated with their cancer, for example, cigarette smoking. Assessment of pulmonary function serves both to identify those patients at a significantly increased likelihood of postoperative morbidity as well as those patients who stand to benefit from preoperative manipulations designed to attenuate those risks. There is no single best test to evaluate pulmonary function in a patient who is slated to undergo pulmonary resection. Also there are no absolute values that contraindicate resection though using a combination of studies it is at least possible to make a judgment as to which patients are at an increased risk for postoperative morbidity or mortality. Preoperative spirometry to measure flows and volumes should be performed. Important measurements include forced expiratory volume in 1 second (FEV1), maximal voluntary ventilation (MVV), diffusing capacity, FEV1/forced vital capacity (FVC) ratio, and the ratio of the residual volume (RV) to total lung capacity (TLC). An FEV1 of less than 40% of predicted has been associated with increased postoperative morbidity and mortality. A reduced diffusing capacity also has been associated with postoperative morbidity. Any assessment of postoperative morbidity and mortality has to take into account the extent of the proposed resection. Often this is difficult to determine and the experience of the surgeon is closely related to the likelihood of pneumonectomy. The surgeon operating on patients with compromised lung function should be experienced in the performance of segmental resections, sleeve resections, and nonanatomic resections, all techniques of lung preservation. Techniques of video-assisted thoracic surgery (VATS) may also be of use in patients with limited pulmonary reserve who require pulmonary resection. There are a number of measures that can be instituted that are designed to attenuate the postoperative risk in patients with compromised lung function. That said, most often it comes down to a judgment on the part of the surgeon based on both objective and subjective factors that is the deciding factor. There are some patients who just are not candidates for pulmonary resection for a variety of reasons that may sometimes be difficult to articulate.
Experience with lung volume reduction surgery in patients with severe end-stage emphysema has changed our approach regarding operation for lung cancer in patients with compromised lung function. Nutritional assessment and therapy and a supervised program of pulmonary rehabilitation may further optimize borderline patients for pulmonary resection. Currently our approach is to be quite aggressive in considering patients with pulmonary malignancies for resection. Rarely is a patient turned down for resection solely on the basis of his/her pulmonary function. At times, the resections have to be somewhat creative and there are a number of intraoperative factors that may contribute to a reduction in postoperative problems. Patients with borderline lung function have to be strongly motivated toward resection. These patients are not the ones to be talked into an operation even if their other treatment options are limited; they really have to be motivated. The major morbidity and potential mortality in these patients occur in the early postoperative period but one must also keep in mind the long-term sequelae of the resection of lung parenchyma in these individuals. Paradoxically there may be some improvement in lung function following pulmonary resection in these patients especially if the lung parenchyma removed receives only a minimal amount of the pulmonary perfusion. Most commonly this occurs with a heterogeneous pattern of emphysema with the upper lobe being the most diseased portion of parenchyma. A preoperative quantitative ventilation-perfusion lung scan is useful in assessing the significance of the loss of lung parenchyma. An estimate of the predicted postoperative FEV1 may be obtained by subtracting the percentage removed by the proposed resection based on the percent of perfusion received by that area of lung parenchyma. A residual FEV1 of less than 800 ml has been associated with an increased risk of postoperative morbidity and mortality but this is entirely dependent on what percent of the predicted FEV1 the 800 ml represents. For instance, in a 50 kg female 800 ml may represent a predicted postresection FEV1 of 60% or more. An algorithm for the preoperative assessment of risk in patients with lung cancer is presented in Figure 4.1.
In addition to pulmonary function studies an assessment of the exercise capability of a patient with compromised lung function may be appropriate. This assessment may range from something as simple as having the patient climb one or two flights of stairs while monitoring oxygen
saturation and pulse rate to formal exercise testing and calculation of maximal oxygen consumption (VO2 max). One can be reasonably certain that a patient who can walk up two flights of stairs can tolerate a lobectomy, a crude assessment to be sure but one that intuitively makes sense. For truly borderline patients, measurement of VO2 max may be the deciding test. A value of less than 15 ml/kg/min has been associated with significantly increased postoperative morbidity and mortality. Patients in this category should be scrutinized closely before deciding to proceed with resection. It is this type of patient who may benefit from a limited resection, albeit a compromise, such as a video-assisted wedge excision if the lesion is small and peripheral. It is highly unlikely that a patient with this severe degree of pulmonary compromise could tolerate a pneumonectomy. Other parameters suggesting high risk include PCO2 >45 torr and elevated pulmonary artery pressures.
saturation and pulse rate to formal exercise testing and calculation of maximal oxygen consumption (VO2 max). One can be reasonably certain that a patient who can walk up two flights of stairs can tolerate a lobectomy, a crude assessment to be sure but one that intuitively makes sense. For truly borderline patients, measurement of VO2 max may be the deciding test. A value of less than 15 ml/kg/min has been associated with significantly increased postoperative morbidity and mortality. Patients in this category should be scrutinized closely before deciding to proceed with resection. It is this type of patient who may benefit from a limited resection, albeit a compromise, such as a video-assisted wedge excision if the lesion is small and peripheral. It is highly unlikely that a patient with this severe degree of pulmonary compromise could tolerate a pneumonectomy. Other parameters suggesting high risk include PCO2 >45 torr and elevated pulmonary artery pressures.
Some patients may undergo invasive staging prior to pulmonary resection. The decision to perform mediastinoscopy may be based on CT scan findings of enlarged mediastinal lymph nodes and the results of the PET scan. The criteria for defining “enlarged” vary and the sensitivity and specificity of the technique vary depending on the size that is set. We perform mediastinoscopy when lymph nodes greater than 1.5 cm in size are seen on the CT scan. Others perform mediastinoscopy on all patients prior to pulmonary resection recognizing that the majority of procedures will reveal only nodes without evidence of metastatic disease. Mediastinal nodes that are positive on PET scan mandate the need for mediastinoscopy unless there is such bulky adenopathy seen on CT scan that tissue confirmation would be redundant. EBUS assessment of the mediastinum with selective needle aspiration biopsy also is being used more frequently to interrogate the mediastinum.
Whether mediastinoscopy is used selectively or routinely, the key point is accurate staging of the mediastinum in the patient with lung cancer. If mediastinoscopy is performed, it is important that lymph node material be obtained for pathologic examination. Accurate mediastinal staging mandates at the least mediastinal lymph node sampling at thoracotomy or, preferably, complete systematic lymph node dissection. The problem with lymph node staging alone is the issue of how lymph nodes are chosen to be sampled, a problem not present when a complete lymph node dissection is carried out. Mere palpation of a node or an assessment of nodal size will miss nodes harboring intranodal or microscopic disease. It is not clear what percentage of nodal disease is missed with a staging procedure because of the variability in the selection of nodes to be sampled and the lack of a study where nodes are first sampled followed by a complete lymph node dissection. From our own experience 10% to 20% of resections where mediastinal lymph node disease is not suspected result in positive lymph nodes being identified by the pathologist. An operation without lymph node staging information must be considered incomplete. Accurate staging allows the surgeon to discuss prognosis realistically with the patient and allows the patient the opportunity to either participate in a trial of postoperative adjuvant therapy or be evaluated for treatment outside of a protocol setting. With prospective randomized clinical trials demonstrating improved survival of patients with completely resected N1 or N2 disease treated with postoperative adjuvant chemotherapy, the quality and completeness of the intraoperative staging has become even more important.
Despite the bias on the part of most physicians that postoperative radiation therapy is of value in resected patients who are found to have either N1 or N2 disease there are no prospective data that demonstrate a survival advantage in patients so treated. One prospective randomized trial of postoperative radiation therapy versus no further treatment for resected patients with squamous cell carcinoma demonstrated a significant reduction in local recurrence but absolutely no difference in survival. A randomized trial conducted by the collaborative efforts of the national cooperative cancer groups compared postoperative chemotherapy (cisplatin, VP-16) and radiation therapy with radiation therapy alone. This trial required that patients either have mediastinoscopy performed or have a completely negative CT scan in addition
to mandating what lymph nodes had to be sampled at the time of thoracotomy. The results of this trial showed no advantage for the combined radiation therapy and chemotherapy arm over radiation therapy alone and no significant difference when compared to historic controls. Interestingly, there did appear to be a survival advantage in the subgroup of patients who had mediastinal lymph node dissection as opposed to lymph node sampling only.
to mandating what lymph nodes had to be sampled at the time of thoracotomy. The results of this trial showed no advantage for the combined radiation therapy and chemotherapy arm over radiation therapy alone and no significant difference when compared to historic controls. Interestingly, there did appear to be a survival advantage in the subgroup of patients who had mediastinal lymph node dissection as opposed to lymph node sampling only.
Long-term survival following pulmonary resection depends both on characteristics of the primary tumor (T stage) and presence or absence of lymph node disease (N stage). Any analysis of survival is greatly dependent on how thoroughly the lymph nodes are staged, as discussed above. These data, accumulated from studies performed by the LCSG, are particularly enlightening because of the stringent requirement for nodal staging that was mandated for entering patients into these studies. Thus, when a patient was staged as N1 we can be assured that was an accurate staging evaluation since the mediastinal lymph nodes would have been sampled and found to be free of tumor.
INTRODUCTION TO RIGHT-SIDED RESECTIONS
There are a number of significant anatomic features specific to right-sided pulmonary resections. The right main pulmonary artery is relatively long and courses posterior to the superior vena cava and traverses the carina. This extra length of the artery, at times, is an advantage for some proximal lesions that in a similar location on the left side would not be resectable because of the short length of the left main pulmonary artery relative to the bifurcation. The distance between the carina and the origin of the right upper lobe bronchus usually is less than 2 cm and the carina is readily mobilized from the right side. Access to the proximal left main stem bronchus is significantly easier from the right side compared to the left side where the aortic arch limits access both to the origin of the left main bronchus and the carina. Mobilization of the carina is not possible from the left chest and even visualization of the carina from the left is difficult. Carinal resections are preferentially performed through a right thoracotomy or, at times, through a median sternotomy.
The superior mediastinum, the space accessed by the mediastinoscope, is well visualized from the right side. The area bounded by the azygous vein (inferior), the trachea (posterior), the subclavian vein (superior), and the superior vena cava (anterior) delineates this compartment whose lymph node-bearing contents may be removed en bloc from the right side. No such access exists on the left side where the left paratracheal nodes are relatively inaccessible because of the location of the aortic arch. As mentioned above, with the access afforded to the carina from the right side it follows that the subcarinal space is readily dissected for lymph node removal. The subcarinal space also is easily accessed on the left side as well.
On the right side the azygous vein is an important anatomic landmark. The vein courses from posterior to anterior across the main stem bronchus to drain into the superior vena cava. Just inferior to where it crosses the main bronchus is the origin of the upper lobe bronchus, a key anatomic feature. Rarely is it necessary to divide the azygous vein but it may be taken with impunity if involved by tumor or limits access to the lymph node-bearing area.
In assessing resections from the right side the upper lobectomy probably is the most straightforward resection though the location of the posterior segmental arterial branch may, at times, be problematic. Right lower lobectomy is complicated by the location of the middle lobe artery and bronchus and the middle lobectomy is considered difficult by some because of the minor fissure.
 SURGICAL TECHNIQUE
SURGICAL TECHNIQUERight Upper Lobectomy
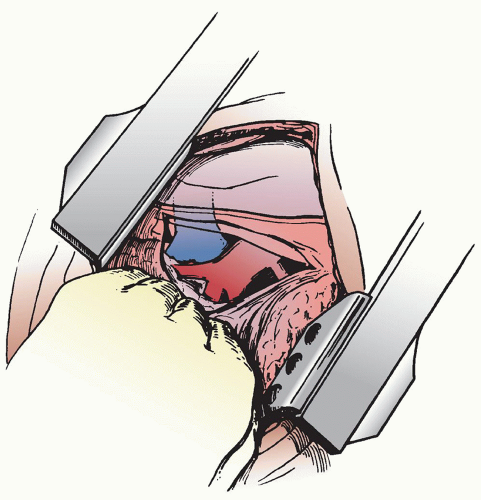
Right upper lobectomy is the prototypical pulmonary resection and is a good starting point for the trainee just starting out in pulmonary surgery. The long right main pulmonary artery with the apical-anterior branch and the discrete takeoff of the upper lobe bronchus makes this an ideal resection. The so-called truncus anterior, the apical-anterior branch of the pulmonary artery, also facilitates segmental resections of the right upper lobe. Once this branch is divided the segmental bronchi are easily visualized.
A left endobronchial double-lumen tube is placed to allow for single-lung ventilation so that the right lung is collapsed for the lobar resection.
With the patient positioned on the left side (left lateral decubitus position), the chest is entered through either a standard posterolateral thoracotomy incision or a vertical axillary muscle-sparing incision. The chest is entered through the fifth intercostal space for the posterolateral incision or the fourth intercostal space for the more anterior muscle-sparing incision. The hilum and mediastinum are palpated to assess the extent of involvement and determine resectability. Access incisions for VATS lobectomy are described in detail in the chapter dealing with VATS pulmonary resection but a small utility incision usually is made anteriorly not only to facilitate the dissection but also to allow for removal of the lobe from the chest. Whether done by a VATS approach or open thoracotomy, the dissection of the pulmonary hilum essentially is the same in that the superior pulmonary vein, the pulmonary arterial branches, and the lobar bronchus must all be taken individually.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree