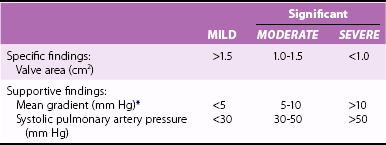
Chapter 17 Unlike other heart valve diseases, mitral stenosis (MS) remains, in most cases, a consequence of rheumatic fever. 1 The main mechanism of stenosis is commissural fusion. Posterior leaflet thickening and restriction are almost constant but have limited hemodynamic consequences. Thickening and rigidity of the anterior leaflet and/or the subvalvular apparatus can also contribute to stenosis ( Figure 17-1). 2 Commissural fusion explains why the area of the mitral orifice is relatively constant in severe stenosis, whereas it may vary according to flow conditions once commissures have been opened after balloon mitral valvotomy (BMV). 3 FIGURE 17-1 Severe mitral stenosis. Degenerative mitral annular calcification occurs frequently in the elderly. It has few or no hemodynamic consequences in most patients, but obstruction may appear over time. 4 Significant stenosis seldom occurs and is related to restriction of both leaflets due to extensive calcification, without commissural fusion. The predominance of rheumatic etiology explains why the prevalence of MS has decreased in industrialized countries. 1 However, it still accounts for approximately 10% of native valve diseases and affects young immigrants or old patients. 5 Conversely, the prevalence of rheumatic heart disease remains sustained in developing countries, being estimated as between 1 and 7 per 1000 in children according to clinical data, and 10 times higher with systematic echocardiographic screening.6,7 The first consequence of MS is the increase in diastolic mitral pressure gradient, which depends on mitral valve area but also on other factors such as transvalvular flow and heart rate. 8 For a given valve area, mean mitral gradient increases when cardiac output increases or when tachycardia reduces the length of the diastolic filling period. Atrial contraction contributes to the increase of transmitral flow in end-diastole and, therefore, of mitral gradient. Severe MS may thus be associated with low mitral gradient in patients with low cardiac output, in particular those who are in chronic atrial fibrillation. The other consequences of MS are blood stasis in the left atrium and, upstream, the decrease in systolic pulmonary vein flow. The severity of blood stasis can be assessed with the use of Doppler echocardiography from the intensity of left atrial spontaneous echo contrast and the decrease in flow velocities in the left atrial appendage in patients in sinus rhythm. Blood stasis and left atrial appendage flow velocities are considerably impaired when atrial fibrillation occurs, thereby increasing the risk of thrombosis. The left atrial appendage is the most common location of left atrial thrombus. 9 Thrombus formation may also be favored by local abnormalities of hemostasis, in particular the increase in fibrinopeptide A and thrombin–antithrombin III complex. 10 A constant mechanism of pulmonary hypertension in MS is the passive rise in pulmonary artery pressure following the increase in left atrial pressure. In addition, pulmonary hypertension can be worsened in certain patients by an increase in pulmonary vascular resistance, which determines a gradient larger than 10 mm Hg between diastolic pulmonary pressure and left atrial pressure. Increased pulmonary vascular resistance involves vasoconstriction and structural changes of the pulmonary arterial wall and explains the possibility of persistent pulmonary hypertension after intervention in MS. 11 Vasoconstriction seems to involve endothelium-dependent vascular tone regulation, as shown by the decrease in pulmonary vascular resistance after inhaled nitric oxide and the probable synthesis of endothelin-1 in the pulmonary circulation.11,12 The sequence of histologic changes in pulmonary hypertension due to MS is characterized initially by medial thickening in muscular arteries and arterioles, followed by intimal thickening. 13 These changes are likely to be reversible with a decrease in pulmonary pressures. More severe pulmonary hypertension is associated with fibrinoid necrosis and arteritis, loss of smooth muscle cell nuclei, fibrin deposition in the arterial wall, and the presence of inflammatory cells. The pathologic hallmark of end-stage, irreversible pulmonary hypertension is the plexiform lesion, which consists of aneurysmal dilation of the arterial wall with a plexus of glomus-like, thin-walled channels branching to join with adjacent capillaries. Nonspecific parenchymal changes in severe pulmonary hypertension include pulmonary hemosiderosis and cholesterol granuloma formation. In a multivariate analysis comprising 744 patients with severe MS, factors associated with pulmonary vascular bed gradient were mitral gradient, left ventricular end-diastolic pressure, mitral valve area, and a history of chronic pulmonary disease. 14 These numerous factors explain the wide range in pulmonary pressures for any given degree of MS. Left ventricular size is generally normal or moderately reduced in MS. Although left ventricular contractility typically is normal in isolated MS, forward stroke volume may be reduced because of low filling volumes across the stenotic mitral valve. In addition, left ventricular ejection fraction is impaired in 5% to 10% of patients with MS in the absence of another cause, in particular other valve or coronary artery disease. 15 This impairment does not seem to be explained by abnormal loading conditions because left ventricular dysfunction generally persists after the relief of MS. Hemodynamic changes during exercise provide additional insights into the multiple factors interacting with the severity of the stenosis to determine its repercussions. The increase in transmitral gradient at exercise is the consequence of the shortening of the diastolic filling period, and it causes an upstream increase in pulmonary artery pressure. However, changes in mitral gradient and pulmonary artery pressure are highly variable for a given degree of stenosis. 16 This heterogeneity may be explained by differences in the evolution of stroke volume during exercise and by differences in atrioventricular compliance.17,18 An increase in stroke volume at exercise, as also occurs in normal patients, is associated with an increase in mitral valve area during exercise in patients who have a moderate impairment of valve anatomy. Conversely, in patients with a severe impairment of valve anatomy, stroke volume does not increase or even decreases during exercise. Net atrioventricular compliance is the strongest determinant of left atrial and pulmonary artery pressures at rest, stronger than valve area, gradient, and pulmonary vascular resistance. 19 A low net compliance is mainly the consequence of a low compliance of the left atrium and is also associated, even more than at rest, with a higher pulmonary artery pressure at exercise and more severe symptoms. 18 The search for comorbidity is important in elderly patients, who account for a growing proportion of patients with MS in developed countries.20,21 Auscultation reveals a loud first heart sound, an opening snap in early diastole just after the second heart sound, followed by a holodiastolic rumbling murmur that decreases in intensity with time and increases in end-diastole in patients in sinus rhythm ( Figure 17-2). The murmur is often difficult to identify because it is localized and of low acoustic frequency. Thus, careful auscultation is needed, using the bell of the stethoscope in different points around the apex with the patient in the left lateral decubitus position. FIGURE 17-2 Correspondence between hemodynamics and auscultation of mitral stenosis. The loudness of the murmur depends on the intensity of the transmitral gradient. A loud murmur with a thrill suggests severe stenosis. Conversely, a low-intensity murmur does not exclude severe stenosis in patients with low cardiac output. The duration of the interval between the second aortic sound and the opening snap is shortened in severe stenosis because increased left atrial pressure causes earlier opening of the mitral valve. 8 The intensity of the first sound and the opening snap may be diminished in cases of extensive calcification that limits leaflet motion. The first abnormality of the cardiac silhouette on chest radiography is left atrial enlargement, characterized by left atrial double density and prominence of the left atrial appendage. Pulmonary hypertension causes dilation of the pulmonary artery trunk and branches. Heart size is normal at this stage. Severe chronic MS leads to right ventricular and right atrial enlargement and cardiomegaly. Pulmonary vascular redistribution and, later, interstitial edema are radiographic signs of elevated left atrial pressure ( Figure 17-3), which are often seen even in patients with moderate symptoms and without clinical signs of heart failure. Alveolar edema is a sign of acute hemodynamic decompensation. Transverse chest radiograph is useful to diagnose right ventricular enlargement, mild pleural effusion, and mitral valve calcification, which is detected by fluoroscopy with a higher sensitivity. FIGURE 17-3 Chest radiograph in a patient with mitral stenosis. Echocardiography is the cornerstone in the evaluation of suspected or known MS and is used to confirm the diagnosis, evaluate the severity and consequences of valve lesions, and assess valve anatomy and associated diseases. 22 Echocardiographic techniques are detailed in Chapter 6. A parasternal short-axis view enables planimetry to be performed, which is the reference measurement of mitral valve area. 23 Its advantage is that it is the only direct measurement of valve area, thereby being independent of loading conditions and associated heart diseases. However, technical expertise is needed to scan the mitral valve apparatus to position the measurement plan on the leaflet tip. Positioning the measurement plan can be facilitated with the use of three-dimensional (3D) echocardiography, which improves reproducibility in this setting, particularly when performed by less experienced echocardiographers.24–26 Planimetry is also useful during balloon mitral valvotomy to monitor the procedure and immediately after balloon mitral valvotomy, when it is the most reliable technique. However, planimetry may be difficult, even not feasible, in the case of an irregular and heavily calcified orifice or in patients with poor echogenicity. Besides planimetry, the parasternal short-axis view assesses commissural fusion. This assessment is of particular importance to differentiate rheumatic MS from MS of other etiologies, in particular degenerative MS, in which commissures are not fused, and to determine the feasibility of balloon or surgical valvotomy. The assessment of commissural opening is also an additional indication of the efficacy of balloon mitral valvotomy during and after the procedure as well as during late follow-up. The assessment of commissural opening is more accurate with 3D than with two-dimensional (2D) echocardiography. 26 The pressure half-time method is generally easier to perform and is therefore widely used. However, it may be misleading in cases of aortic regurgitation, in abnormal compliance of cardiac chambers, and immediately after balloon mitral valvotomy. 23 The most important discrepancies with planimetry are observed in patients older than 60 years and in those in atrial fibrillation. 27 The use of the continuity equation to assess valve area is not valid in cases of associated significant mitral or aortic regurgitation. The method’s accuracy and reproducibility are limited given the number of measurements involved. 23 The proximal isovelocity surface area is technically demanding and requires multiple measurements. Its accuracy can be improved with the use of M-mode echocardiography. 28 The consistency of results of planimetry, pressure half-time method, and gradient should always be checked, with the limitations of the different measurements kept in mind.23,29,30 The continuity equation and proximal isovelocity surface area are not used routinely but may be useful when other methods lead to uncertain or discordant findings. Mitral valve area is considered significant when valve area is less than 1.5 cm2 and severe when it is 1 cm2.23,29,30 A valve area of 1.5 cm2 corresponds to the value above which hemodynamics are not affected at rest. The interpretation of valve area should take body size into account, even if no definite value indexed on body surface area is advised in guidelines ( Table 17-1). TABLE 17-1 Classification of Mitral Stenosis Severity: European Association of Echocardiography/American Society of Echocardiography Recommendations for Clinical Practice *At heart rates between 60 and 80 beats/min and in sinus rhythm. Adapted from Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23. Mitral valve resistance has been proposed as an alternative measurement of the severity of valve obstruction.23,31 Although it is a good predictor of pulmonary artery pressure, mitral valve resistance has not superceded valve area as the marker of MS severity. The analysis of the morphology of valve leaflets and subvalvular apparatus using two-dimensional echocardiography is a key feature of diagnosis of MS and has important implications for the potential of progression and, in particular, the choice of the most appropriate intervention when needed. 23 Echocardiographic evaluation assesses leaflet thickening (significant if greater than or equal to 5 mm), leaflet mobility on the long-axis parasternal view, and calcification, which is best confirmed by fluoroscopic examination. The parasternal short-axis view is paramount not only for planimetry, but also to evaluate the homogeneity of the impairment of the mitral orifice, with focus on commissural areas ( Figure 17-4). Long-axis parasternal and apical views enable impairment of subvalvular apparatus (thickening and/or shortening of chordae) to be assessed, although it tends to be underestimated in comparison with anatomic findings. FIGURE 17-4 Mitral stenosis with calcification of the external commissure. The severity of valvular and subvalvular involvement usually is described by a combined score. The Wilkins score grades each of the following components of mitral apparatus from 1 to 4: leaflet mobility, thickness, calcification, and impairment of subvalvular apparatus ( Table 17-2). 32 The final scores range from 4 to 16. An alternative approach, described by Cormier et al,33,34 is to assess the whole mitral valve anatomy according to the best surgical alternative, which leads to classification in one of three groups ( Table 17-3). TABLE 17-2 Assessment of Mitral Valve Anatomy According to the Wilkins Score * *The total score is the sum of the four items and ranges between 4 and 16. From Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988;60:299–308. TABLE 17-3 Assessment of Mitral Valve Anatomy According to the Cormier Score Data from Cormier B, Vahanian A, Michel PL, et al. Evaluation by two-dimensional and Doppler echocardiography of the results of percutaneous mitral valvuloplasty. Arch Mal Coeur Vaiss 1989;82:185-191; and Vahanian A, Michel PL, Cormier B, et al. Results of percutaneous mitral commissurotomy in 200 patients. Am J Cardiol 1989;63:847–52. These two scoring systems share limitations related to the lack of a detailed location of calcification and leaflet thickening, particularly particular in relation to commissural areas, which are likely to influence the results of balloon mitral valvotomy.35–39 In addition, they both tend to underestimate the weight of subvalvular apparatus impairment. 40 Other scoring systems include a more detailed approach, some of which aim to achieve a better prediction of the results of balloon mitral valvotomy.23,41 However, in addition to concerns related to their reproducibility, they still lack validation in large prospective series and so are not widely used in current practice. Thus, no comparative evaluation of different scoring systems enables a particular one to be recommended. 42 In addition, it is unlikely that a single scoring system could combine reproducibility and accurate prediction of the results of mitral valvotomy. It is advised that the echocardiographer use a method with which he or she is familiar and include the assessment of valve morphology among other clinical and echocardiographic findings. The quantitation of left atrial enlargement using time-motion measurement is the most widely used but lacks accuracy. The estimation of left atrial area or, better, volume using two-dimensional echocardiography is preferred ( Figure 17-5).43,44 FIGURE 17-5 Left atrial volume measurement. The quantitation of associated MR should combine different semiquantitative and quantitative measurements and check their consistency. An accurate evaluation using quantitative methods is of particular importance for moderate regurgitation because it may have important implications in the choice of the type of intervention. 45 Functional tricuspid regurgitation is caused by enlargement of right cavities secondary to pulmonary hypertension without rheumatic lesions of the valve. The quantitation of tricuspid regurgitation is less well established than that of left-sided heart valve regurgitation and is highly dependent on loading conditions. The diameter of the tricuspid annulus seems to be a better marker of the persistence of severe tricuspid regurgitation after the treatment of MS.45,46 However, further standardization of its measurement is needed. Rheumatic tricuspid disease is less frequent. It is characterized by thickening and decreased mobility of tricuspid leaflets and may combine stenosis and regurgitation. Transesophageal echocardiography (TEE) has a much higher sensitivity than transthoracic echocardiography to detect left atrial thrombus, in particular that located in the left atrial appendage. TEE is therefore mandatory before BMV. TEE is also useful to assess left atrial spontaneous echo contrast, which is a strong predictor of thromboembolic risk in patients with MS. 47 Semisupine bicycle ergometry enables hemodynamic changes to be sequentially assessed for increasing workload, in particular mean mitral gradient and estimated systolic pulmonary artery pressure ( Figure 17-6). It is useful in patients whose symptoms are equivocal or are discordant with the severity of MS. However, thresholds of mitral gradient and pulmonary artery pressure, as stated in guidelines to consider intervention in asymptomatic patients, rely on low levels of evidence and are frequently achieved in practice. 23 FIGURE 17-6 Exercise echocardiography in mitral stenosis. Dobutamine stress echocardiography, although less physiologic than exercise echocardiography, increases mean gradient and systolic pulmonary artery pressure. 23 It has been shown to have a prognostic value in one study. 48 Preliminary reports suggest that cardiac magnetic resonance and multislice computed tomography are reliable alternate techniques for performing planimetry of the mitral valve.49,50 Although the availability of such techniques is limited, they may be helpful when echocardiographic imaging is of poor quality. There is now little interest in the use of right and left heart cardiac catheterization to calculate mitral valve area using the Gorlin formula. The validity of the Gorlin formula is questionable when cardiac output is decreased and immediately after balloon mitral valvotomy. 51 Thus, invasive evaluation of the severity of MS is justified in only cases in which echocardiography results are inconclusive.23,29,30 The development of MS takes many years following acute rheumatic fever. It is difficult to evaluate the course of the disease because rheumatic fever is not always diagnosed, is often subject to recurrences, and is subject to highly variable evolution according to the country considered. A majority of patients with initial rheumatic carditis eventually have chronic rheumatic valve disease. A prospective study using echocardiography identified three risk factors for progression toward chronic rheumatic valve disease: the severity of carditis, recurrences of acute rheumatic fever, and mother’s low educational level. 52 The course of the disease is particularly rapid in countries where rheumatic fever is endemic, leading to severe MS in young adults, adolescents, and even children. Conversely, MS frequently occurs in adults older than 50 years in western countries. This is illustrated by series of balloon mitral valvotomy, in which mean patient age is around 30 years in Asia and North Africa but between 40 and 60 years in series from Europe and the United States. 53 The progression of MS has been evaluated in series involving serial hemodynamic or echocardiographic evaluations.54–56 They are subject to bias because all of them were retrospective and included a limited number of patients. They reported an average decrease of 0.01 cm2/year, although this figure reflects a mix between patients in whom valve area remains stable, accounting for between one and two thirds, and patients experiencing progression with an annual decrease in valve area ranging between 0.1 and 0.3 cm2. Impairment of valve anatomy (Wilkins score ≥8) and a peak mitral gradient of 10 mm Hg or greater were identified as predictors of a more rapid progression of MS. 55 A nonrandomized study suggested that the use of statin drugs may slow the progression of MS. 57 As in other valve diseases, studies on the natural history of MS are frequently old, retrospective, and subject to inclusion bias. Despite these limitations, which may explain differences in estimations, there is an agreement on the poor prognosis of MS when patients become symptomatic, with 10-year survival rates ranging from 34% to 61% and 20-year rates between 14% and 21%.58,59 A later series reported 44% survival at 5 years in patients refusing intervention. 60 Survival is highly influenced by the evolutionary stage of the disease, in particular symptoms and atrial fibrillation. Asymptomatic patients have a 20-year survival exceeding 80%, but approximately half of them become symptomatic after 10 years. 58 Clinical deterioration is sudden in approximately half of patients. The leading cause of death is heart failure, in about 60% of patients, followed by thromboembolic complications in about 20%. 58 Atrial fibrillation is a frequent complication of MS and it is largely related to left atrial enlargement. However, its frequency is only partly related to the severity of stenosis. 61 As in the general population, the frequency of atrial fibrillation is also strongly dependent on patient age. 20 The use of systematic Holter electrocardiogram (ECG) monitoring in one study showed that half of patients with MS in sinus rhythm had atrial arrhythmias although 95% were asymptomatic; 14% of them presented embolic complications. The three predictive factors of atrial arrhythmias were age, left atrial diameter, and valve calcification. 62 Atrial fibrillation considerably worsens the consequences of MS. The lack of atrial contraction and the shortening of the diastolic filling period further impair hemodynamics and may cause acute decompensation such as pulmonary edema. The other consequence is the increase in blood stasis in the left atrium, which increases the thromboembolic risk. The Framingham Heart Study 62a estimated a 17-fold increase in the risk of stroke in patients with atrial fibrillation and MS, compared with a 5-fold increase in risk for atrial fibrillation in the absence of mitral valve disease. Annual linearized risk of thromboembolism in atrial fibrillation without anticoagulant therapy has been estimated to 3.6% for moderate MS and 5.7% for severe MS. The corresponding figures in patients in sinus rhythm were estimated to be 0.25% for moderate MS and 0.85% for severe MS. 60 In cases of atrial fibrillation, most embolic complications originate from left atrial thrombosis, which is located in the left atrial appendage. Embolic events are cerebral in location in 60% to 70% of cases, leave sequelae in 30% to 45% of cases, and are prone to recurrence. 63 Left atrial spontaneous echo contrast as assessed by TEE plays a particularly important role in risk stratification for thromboembolic risk in MS. 64 Primary prevention relies on adapted antibiotic treatment of streptococcal pharyngitis. Secondary prevention is based on the use of continuous antibiotic therapy. 65 Although painful, intramuscular injection of benzathine penicillin every 3 weeks has the advantage of a better compliance than daily oral treatment, in particular in young patients and in developing countries. Antibiotic prophylaxis of rheumatic fever treatment is advised for up to 25 years in patients with rheumatic carditis (see Chapter 9). Once rheumatic valve disease has occurred, no medical treatment has been shown to be able to slow the progression of MS. The prevention of infective endocarditis has been recently reoriented toward reduced indications for antibiotic prophylaxis, which is no longer advised in native heart valve diseases. On the other hand, the importance of general hygiene measures is stressed, particularly dental and cutaneous hygiene.66,67 Beta-blockers are particularly useful in pregnant women, enabling a dramatic decrease in mean gradient and pulmonary artery pressure in most cases. However, beta-blockers do not seem to improve exercise tolerance in MS.68,69 In patients with MS and atrial fibrillation, restoration of sinus rhythm is superior to rate control as regards indices of functional capacity and quality of life. 70 When atrial fibrillation cannot be converted in sinus rhythm, rate control is obtained using digitalis and/or beta-blockers. Unlike in patients with nonvalvular atrial fibrillation, there are no randomized trials on the efficacy of anticoagulant therapy in patients with MS with or without atrial arrhythmias. Permanent or paroxysmal atrial fibrillation is a class I indication for oral anticoagulation, regardless of stenosis severity, in American College of Cardiology/American Heart Association (ACC/AHA) as well as European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines.29,30 In a retrospective study, oral anticoagulation decreased the annual risk of thromboembolism in patients with MS and atrial fibrillation from 5.7% to 1.0% for severe MS and from 3.6% to 0.9% for moderate MS. 60 In patients with MS in sinus rhythm, the annual risk of thromboembolism decreased from 0.85% to 0.10% for severe MS and from 0.25% to 0.10% for moderate MS. 60 Given the risk of bleeding inherent to oral anticoagulation, the analysis of risk and benefits does not support systematic anticoagulant therapy in patients with MS in sinus rhythm. Anticoagulant therapy using vitamin K antagonists is advised in selected patients with MS in sinus rhythm who are at high risk for thromboembolic events according to the following criteria. Prior embolism and left atrial thrombus are class I recommendations for oral anticoagulation in ACC/AHA and ESC/EACTS guidelines. Dense spontaneous echo contrast and enlargement of the left atrium are class IIa recommendations in ESC/EACTS guidelines and class IIb recommendations in ACC/AHA guidelines. Target international normalized ratio is 2.5 (i.e., a range between 2.0 and 3.0). Aspirin or other antiplatelet drugs alone are not valid alternatives to decrease thromboembolic risk in patients with MS. Newer anticoagulants, such as dabigatran, rivaroxaban, and apixaban, cannot be recommended at present because patients with MS were excluded from trials comparing these drugs with warfarin for the prevention of embolism in atrial fibrillation. A randomized trial found benefit in a combination of an antiplatelet drug with low-dose oral anticoagulation over conventional anticoagulation, but this finding requires further confirmation. 71 Pharmacologic or electric cardioversion should be attempted in patients with nonsevere MS who have persistent atrial fibrillation. In patients with severe MS, cardioversion should be postponed after the intervention on the mitral valve, in most cases, because restoration of sinus rhythm is unlikely to be sustained in the absence of intervention. 30
Rheumatic Mitral Valve Disease
Pathophysiology
Mechanisms of Valve Obstruction
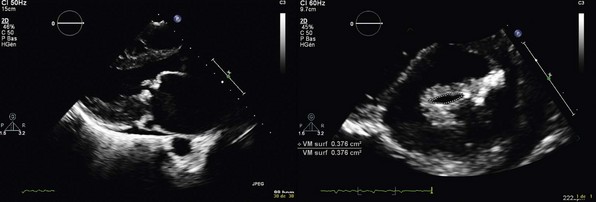
Transthoracic echocardiography: parasternal long-axis (left panel) and short-axis (right panel) views. Valve area is 0.4 cm2. Leaflet tips are thickened but anterior leaflet is pliable. Subvalvular apparatus is severely impaired with shortened and fused chordae. Both leaflets are moderately thickened with a pliable anterior leaflet on the long-axis view and fusion of both commissures on the short-axis view. There is a dense nodule of the internal commissure.
Hemodynamic Consequences of Mitral Stenosis
Mitral Gradient
Left Atrium
Pulmonary Circulation
Left Ventricle
Exercise Physiology
Clinical Presentation
History
Physical Examination
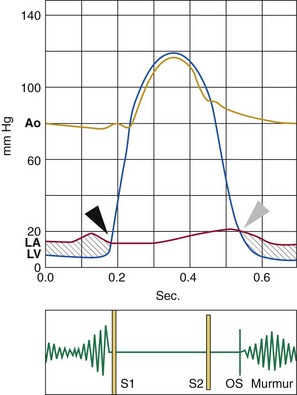
The diastolic rumbling murmur corresponds to the pressure gradient between the left atrium and left ventricle (in gray). Murmur intensity decreases progressively and is reinforced in end-diastole with atrial contraction. Intensity of the first heart sound (S1) is increased. The interval between the second heart sound (S2) and the opening snap (OS) decreases as mitral stenosis becomes more severe. Black arrowhead indicates mitral valve closure and gray arrowhead indicates mitral valve opening. Ao, Aortic pressure; LA, left atrial pressure; LV, left ventricular pressure.
Chest Radiography and Electrocardiography
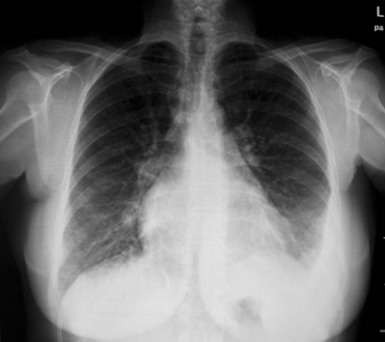
Left atrium and pulmonary trunk are enlarged. There is marked pulmonary congestion with interstitial pulmonary edema and left pleural effusion.
Echocardiography
Assessment of Severity
Assessment of Valve Morphology
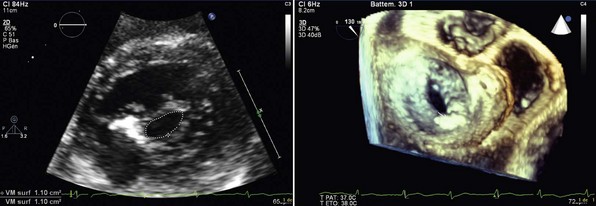
Left panel, Two-dimensional echocardiography, parasternal short-axis view. Right panel, Three-dimensional echocardiography, view from the left atrium.
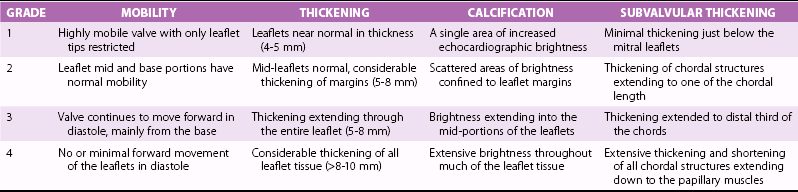
ECHOCARDIOGRAPHIC GROUP
MITRAL VALVE ANATOMY
1
Pliable noncalcified anterior mitral leaflet and mild subvalvular disease (i.e., thin chordae ≥10 mm long)
2
Pliable noncalcified anterior mitral leaflet and severe subvalvular disease (i.e., thickened chordae <10 mm long)
3
Calcification of mitral valve of any extent, as assessed by fluoroscopy, whatever the state of subvalvular apparatus
Consequences of Mitral Stenosis
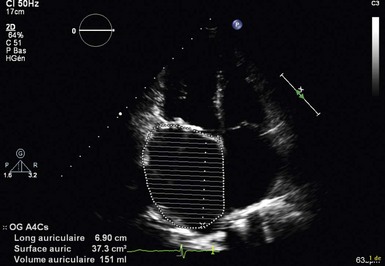
Apical four-chamber view of the left atrium with traced contour to determine left atrial area and volume. Left atrial area is 37 cm2, and volume is 151 mL.
Mitral Regurgitation
Associated Lesions
Thromboembolic Risk
Stress Testing
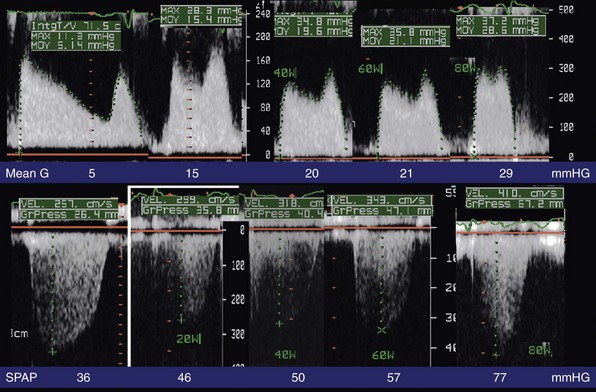
Monitoring of mitral gradient (upper panel) and pulmonary artery pressure (lower panel) at rest and at (from left to right) 20, 40, 60, and 80 watts with bicycle exercise in a semisupine position. Mean G, Mean mitral gradient; SPAP, systolic pulmonary artery pressure. (Courtesy Dr Brochet.)
Other Noninvasive Investigations
Cardiac Catheterization
Natural History
Onset and Progression of Valvular Lesions
Clinical Outcome without Intervention
Complications
Medical Therapy
Prevention of Rheumatic Fever
Treatment of Symptoms
Prevention of Thromboembolism
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









