Complementary and alternative medical (CAM) therapies are commonly used by patients for the treatment of medical conditions spanning the full spectrum of severity and chronicity. The use of alternative remedies, both herbal and others, for conditions lacking effective medical treatment, is on the increase. Included within this categorization, arrhythmic disease–absent effective catheter-based therapy or with medical therapy limited by the toxicities of contemporary antiarrhythmic agents is frequently managed by patients with CAM therapies without their practitioner’s knowledge and in the face of potential herb-drug toxicities. This study reviews 9 CAM therapies: 7 individual herbal therapies along with acupuncture and yoga that have been studied and reported as having an antiarrhythmic effect. The primary focuses are the proposed antiarrhythmic mechanism of each CAM agent along with interactions between the CAM therapies and commonly prescribed medical therapy for arrhythmia patients. We stress persistent vigilance on the part of the provider in discussing the use of herbal or other CAM agents within the arrhythmia population.
Contemporary medical literature is sparse regarding the safety and efficacy of commonly used nontraditional arrhythmia therapies. In contrast, a cursory Internet search reveals an astounding number of websites discussing an impressive array of available alternative therapies for arrhythmias. Many of these complementary and alternative medical (CAM) remedies have antiarrhythmic properties similar to prescription antiarrhythmic agents and if taken inappropriately, particularly in combination with prescription antiarrhythmic agents, may prove harmful. Moreover, many CAM therapies can alter the metabolism of other evidence-based heart failure or antiarrhythmic agents resulting in avoidable toxicity and adverse clinical events. This study reviews the efficacy and safety of CAM therapies (herbal, Yoga, and acupuncture) reported as having antiarrhythmic activity or efficacy in PubMed. Our focus is CAM therapies with known and described antiarrhythmic properties followed by the safety and drug-drug interactions of commonly used agents. The agents discussed are far from all inclusive and only those identified through the available medical literature are included.
CAM Agents With Reported Antiarrhythmic Properties
A list of the discussed CAM agents, their proposed mechanisms, and known CAM agent–drug interactions is presented in Table 1 .
| CAM Therapy | Mechanism of Action | Antiarrhythmic Behavior | State of Evidence | CAM/Drug Interactions |
|---|---|---|---|---|
| Acupuncture | Unknown—possible resetting of vagal/sympathetic axis | Unknown—possibly class 2 | Humans—RCT | None |
| Barberry | Reduction in I to | Class 1A/3 | In vitro/animal models | CYP3A4 inhibitor; increases statin, cyclosporine levels among others. |
| Cinchona | Reduction in INa | Class 1 | Scant—extrapolated from quinidine evidence | CYP3A4 inducer; decreases tegretol levels. “Chincronism” |
| Hawthorn | Reduction in IK s and IK r | Class 3 | In vitro/animal models | ↑ Digoxin activity; inhibits thrombox. A2: more bleeding on anticoagulants |
| Khella | Reduction in IK s and IK r | Class 3 | Scant—extrapolated from amiodarone evidence | Multiple; similar to amiodarone |
| Motherwort | Reduction in I f , ICa .L , and IK .r | Class 3 | In vitro/animal models | Increased risk of bleeding on antiplatelets or anticoagulants. |
| Omega-3 PUFA | Unknown—reduction in proarrhythmic fatty acids | Unknown—possibly class 1, 2, or 4 | Humans—RCT | Rare dramatic elevation in INR with coumadin |
| Wenxin Keli | Reduction in INa | Atrial selective class 1 | In vitro/animal models | Unknown—not studied |
| Yoga | Unknown—possible reduction in sympathetic tone | Unknown—possibly class 2 | Humans | None |
Motherwort ( Leonurus cardiaca )
Motherwort ( L cardiaca ) has a long history of use in both European and Asian traditional medicine, dating back to the 15th century, secondary to its sedative and antispasmodic properties. Used by the Greeks for the treatment of anxiety in pregnancy, it acquired its name motherwort or “Mother’s Herb.” Regarding cardiovascular maladies, it has been used for a generic “cardiac debility” and tachycardia or palpitations. Phenylpropanoid glycosides have been detected in multiple preparations of motherwort and appear to play a dominant role in their purported pharmacologic activity. In particular, lavandulifolioside, a phenylpropanoid isolated from motherwort, has been shown to have significant negative chronotropic effects and to prolong the PR, QRS, and QT intervals in rats.
Further work in isolated rabbit, rat, and guinea pig hearts identified the electrophysiologic and related therapeutic effects of motherwort. Prolongation of the activation time constant of I f , antagonism of ICa .L , and reductions in the repolarizing current IK .r were observed with motherwort preparations both at whole organ and single cell level. Such effects at the cellular level explain the reduction in sinus rate (I f ) along with prolongation of the PQ interval (ICa .L , IK .r ) seen at the organ level. Its antiarrhythmic activity is similar to class 3 antiarrhythmic agents with little effect on phase 0 of the action potential, prolongation of phase 2, and a reduction in I f resulting in a slowing of the sinus rate.
Currently no data regarding the efficacy and safety of motherwort for the treatment of palpitations or as an antiarrhythmic agent is available. In similar fashion, little is known about the metabolism of the pharmacologically active components of motherwort. Given its antiplatelet effects, its use should be avoided in any patient on concurrent antiplatelet or anticoagulant therapy and/or those with a bleeding diathesis. As its effects on the cytochrome system remain unknown, it should be used with caution in the setting of drugs whose metabolism is cytochrome dependent with narrow therapeutic windows.
Wenxin Keli
Wenxin Keli is a Chinese herb extract reported to be of benefit in the treatment of cardiac arrhythmias, cardiac inflammation, and heart failure. Wenxin Keli is composed of 5 agents: Nardostachys chinensis Batal extract, Codonopsis , notoginseng, amber, and Rhizoma polygonati . In China, it possesses a licensable indication for premature ventricular contractions, which was included in the 2009 revision of the National Reimbursement Drug List.
Recent animal model work by Burashnikov et al has supported the ability of Wenxin Keli to suppress and prevent atrial fibrillation (AF) in dog models. Within this study, the antiarrhythmic activity of Wenxin Keli occurred through a unique semiselective depression of atrial INa channels and prolongation of the atrial effective refractory period. The clinical efficacy, metabolism, and potential herb-drug interactions in human remains to be studied, although its atrial selectivity makes it a very interesting potential pharmaceutical antiarrhythmic agent.
Cinchona ( Cinchona , Various Species)
Cinchona bark contains a number of quinone alkaloids, primarily quinine, quinidine, cinchonine, and cinchonidine. Quinine and its stereoisomer quinidine are the most familiar and pharmacologically active of these compounds occurring in amounts of 0% to 14% by weight in cinchona bark. The use of cinchona bark for the treatment of malarial infections dates back to the 17th century. Its use as an antiarrhythmic was introduced in 1918 by Walter Frey as the “common alkaloid” of cinchona bark and is still used as the purified class 1A antiarrhythmic quinidine today.
Given that it is the original source of quinidine, any antiarrhythmic effect and possible therapeutic benefit for the treatment of palpitations would be expected to result from the class 1A antiarrhythmic activity (prolongation of phase 0 and the entire action potential) of the quinidine. No data regarding the antiarrhythmic benefit of cinchona are currently available. It is also unknown if cinchona would have the same proarrhythmic effect and increased mortality seen with other class 1 antiarrhythmics when used in patients with previous myocardial infarction.
The only significant medication interaction noted with cinchona is a decrease in systemic levels of carbamazepine through induction of CYP3A4 by way of which it is metabolized. However, such a short list may be secondary to a well-described underreporting of adverse drug interactions with CAM agents. With the active ingredients quinine and quinidine having a much better understood and extensive list of medication interactions, the cautious approach would be to assess for any possible interactions with these 2 agents.
Hawthorn ( Crataegus oxycantha )
Extract from the berries and flowers of the common hawthorn plant ( C oxycantha ) is a popular herbal supplement widely used by herbalists for the treatment of angina, arrhythmia, hypertension, and congestive heart failure. Its use as a cardiovascular agent in European medicine dates to 1st century Greek herbalist Dioscorides and later Swiss physician Paracelsus (1493 to 1541). As a CAM treatment for cardiovascular maladies, it remains in widespread use today.
Hawthorn has positive inotropic and vasodilatory effects and, in related fashion, is thought to increase myocardial perfusion and reduce afterload. Regarding antiarrhythmic effects, Hawthorn extract has been shown to prolong the action potential through an inhibition of the inward potassium channels IK s and IK r . This effect is similar to that of class 3 antiarrhythmic agents and forms the basis of the antiarrhythmic effects described for hawthorn extract. Of note, hawthorn appears to be selectively active for these currents, in contrast to the remaining majority of the class 3 antiarrhythmic agents that have additional β- or calcium channel–blocking properties.
Hawthorn likely enhances the activity of digitalis, although this remains controversial as its method of metabolism is currently unknown, and as a result its concomitant use should be monitored for the development of toxicity. Hawthorn also inhibits the biosynthesis of thromboxane A2, and it could potentially increase the risk of bleeding in patients taking antiplatelets and/or anticoagulant agents. Without additional data on metabolism, safety, and efficacy, clinicians should discourage unsupervised use of hawthorn in patients with congestive heart failure who are taking heart failure medications.
Khella ( Ammi majus )
Khellin, an extract from the fruit of the khella or A majus plant, has long been used for noncardiac ailments in its native region of North Africa. The discovery of the antiarrhythmic properties of khellin resulting in its eventual purification to pharmaceutical grade amiodarone was made by the Lebanese physiologist Gleb von Anrep while working in Cairo circa 1946 as detailed by Dr. Arthur Hollman in the text Cardiology from Nature . A technician in his laboratory was taking khella for an unrelated issue and found a coincident relief from his longstanding anginal symptoms. This observation led to the isolation of khellin, the active component of khella, by Dr. Anrep. Amiodarone was eventually developed in 1961 at the Labaz Co. in Belgium, by chemists Tondeur and Binon, from preparations of khellin with subsequent popularity in Europe as a treatment for angina pectoris. With additional investigation by Dr. Bramah Singh, the antiarrhythmic properties of amiodarone were fully described making it the first member of a new class of antiarrhythmic agents (eventually becoming class 3).
As would be expected from its role in the development of amiodarone, khella extract has antiarrhythmic properties similar to those of amiodarone. Little clinical data exist for the use of khella either in animals or in humans for antiarrhythmic purposes. It stands to reason that the action, metabolism, medication interactions, side effects, and toxicities of khella are similar to those of amiodarone; however, these presumed similarities are little more than assumptions, as they have not been described clinically or experimentally. Khella has been described to cause a pigmentary retinopathy in wild birds that consume it, not unlike that seen with amiodarone, providing some strength to assumed similarities between itself and amiodarone.
Compared with amiodarone, khella extract is certainly less potent from an antiarrhythmic standpoint but still may possess antiarrhythmic properties along with a risk for currently unknown medication interactions and toxicities. Its consumption and possible drug interactions should be treated the same as amiodarone as we know little about its clinical effects otherwise.
Barberry ( Berberis vulgaris )
Barberry is a shrub that is common to most areas of temperate Europe, Asia, Africa, and Northeastern regions of the United States. Both the barberry fruit and the root are used to make extracts with the root containing a larger proportion of the active alkaloid berberine. Its use dates back over 2,500 years in Ayurvedic and Chinese medicine as a treatment for fever and gastrointestinal disorders. Iran is currently the largest producer of barberry where it is used commonly as a food seasoning and as an antibacterial, antipyretic, antipruritic, and antiarrhythmic agent.
Previous pharmacologic studies on berberine, an isoquinoline alkaloid found in the root and bark of B vulgaris , demonstrated that it possessed potent vasodilatory and antiarrhythmic activity. Its antiarrhythmic activity stems from an associated prolongation of the action potential duration through a dose-dependent inhibition of I to . Such an effect has been shown in animal models and human atrial cells in vitro and is consistent with the antiarrhythmic effects of disopyramide and quinidine, both class 1A agents. The selective nature of the I to blockade with berberine differentiates it from the class 1A agents in that it is not accompanied by inward sodium current inhibition. The resulting prolongation of the action potential is more consistent with class 3 activity and results in longer effective refractory periods. Given these isolated effects on I to , this agent has possible therapeutic potential for Brugada patients as their loss of function in the INa channel results in unopposed I to activity. Berberine may provide a more specific (and possibly more potent) quinidine-like effect on I to and a similar or larger reduction in subsequent ventricular fibrillation and death. Further study is required before advocating the use of berberine in such a fashion.
Currently, no clinical evidence for its efficacy exists but with its similarities to some class 1A and/or 3 antiarrhythmic agents, it is likely to carry some proarrhythmic effects along with any potential efficacy. Moreover, berberine has been shown to inhibit CYP3A4 similar to the action of grapefruit. Such inhibition will increase blood levels of statins, cyclosporine, calcium channel blockers, midazolam, estrogen, and terazosin. The action of these medications is potentiated by increased bioavailability, which potentially can result in dangerous hypotension, myopathy, or liver toxicity. These potential interactions should be discussed with patients taking medications metabolized by the CYP3A4 system, and they should be advised to avoid barberry-derived products.
Omega-3 Fatty Acids
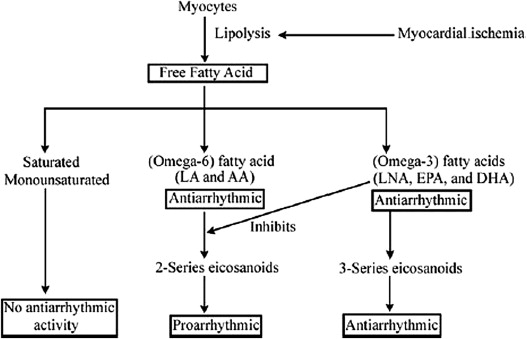
Beyond their use in hyperlipidemia, a number of basic and clinical studies have provided evidence for clinically significant antiarrhythmic properties of omega-3 fatty acids. The most significant data in support of an antiarrhythmic effect of omega-3 fatty acids were found in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico–Prevenzione trial with a significant reduction in the incidence of sudden death for survivors of myocardial infarction treated with omega-3 fatty acids. Numerous additional investigations have supported the benefit of fish oil intake for the reduction in serious ventricular arrhythmias. However, the effect of omega-3 supplementation on AF appears marginal and remains an area of ongoing debate with most data not supporting its efficacy.
The proposed mechanism behind this reduction is an inhibition of the conversion of omega-6 fatty acids to their proarrhythmic cyclooxygenase metabolites ( Figure 1 ). Most experimental studies indicate that omega-3 fatty acids may prevent fatal ventricular arrhythmias, at least in part, by inhibition of voltage-gated sodium channels and maintenance of L-type calcium channels to prevent calcium overload during stress. In addition, omega-3 fatty acids have been shown to prevent or attenuate β agonist–induced arrhythmias in vitro, possibly supporting a β blockade–like effect. However, the primary site of action of omega-3 fatty acids along with the exact mechanism has not been determined and is an area of active research.