Resorption of Gases from the Pleural Space
Yvon Cormier
Steve Provencher
Under normal circumstances, there are no free gases in the virtual space between the parietal and visceral pleura. The pressure in the pleural space is negative in relation to the atmosphere because of the elastic properties of the lungs, which tend to collapse, and of the chest wall, which, at volumes below 75% of total lung capacity, tends to expand. In normal individuals, at functional residual capacity when respiratory muscles of the thoracic wall are in the relaxed state, this pressure is approximately 5 cm H2O lower than that of the surrounding atmosphere. This pressure further decreases during inspiration and can become lower than -100 cm H2O during a Müller maneuver (i.e., maximal inspiratory efforts against a closed glottis). Because of this pressure gradient, any communication between the pleural space and the surrounding structures (bronchi, alveoli, extrathoracic communication through the chest wall) immediately causes gases to enter the pleural space and produce a pneumothorax. When gases enter the pleural space, pressure gradients and the physical laws of gases favor their eventual resorption.
Factors Determining Gas Resorption
Gas resorption from the pleural space is achieved by a simple diffusion from the pleural space into the venous blood. This diffusion, which can occur in both directions, is possible because the pleura and capillary walls are permeable to gases and the partial pressures of gases in the pleural space and those in the venous blood differ. Indeed, no active transport mechanisms for gas resorption exist; the only driving forces that determine gas resorption are pressure gradients.
The rate of gas resorption depends on four variables: the pressure gradients for the gases in the pleural space in relation to the venous blood, the area of contact between the pleural gas and the pleura, the permeability of the pleural surface (e.g., a thickened fibrotic pleura absorbs less than a normal pleura), and the diffusion properties of the gases present in the pleural space. Indeed, the solubility and diffusion properties of different gases vary considerably: oxygen is resorbed 62 times faster than nitrogen (N2), and water vapor (H2O) and carbon dioxide (CO2) equilibrate almost instantaneously, CO2 being 23 times more soluble than O2.
Depending on the clinical situation, a pneumothorax can initially contain room air (i.e., a leak from the outside) or alveolar air (i.e., a leak through the lungs). The alveolar air contains different proportions of CO2, O2, or N2, depending on the ventilation of the patient and the presence of supplemental O2 given to the patient at the time of the leak into the pleural space. If a patient is receiving 100% O2, the pleural gases will be composed mostly of this gas and contain no N2, the slowest gas to be resorbed. Initial gas compositions in a pneumothorax when the air entry is from room air or from alveolar air with the patient breathing room air or 100% O2 are presented in Figure 57-1.
Mechanisms of Gas Pressure Gradients between the Pneumothorax and the Venous Blood
Because the pressure gradients that favor gas resorption are those between the pneumothorax and the venous blood, it is also important to consider the venous blood partial gas pressures. A schematic approach is used here to explain the gas composition in venous blood. Dry room air contains, for all practical purposes, 80% N2 and 20% O2. Other gases (e.g., CO2, argon) are present in minute quantities. The normal atmospheric pressure is 760 mm Hg at sea level (1,031 cm H2O); therefore the partial pressure of N2 in dry room air is 608 mm Hg, whereas that of O2 is 152 mm Hg. When room air enters the alveoli, it gains H2O vapor and CO2 and loses O2; the resulting gas composition is N2 = 571 mm Hg, O2 = 102 mm Hg, H2O = 47 mm Hg, and CO2 = 40 mm Hg. Because the alveoli are in close communication with the atmosphere, the total gas pressure at this level must equal 760 mm Hg. This alveolar gas composition is in contact with the blood at the alveolar capillary level, and gas exchange occurs. The resulting normal arterial gas composition is PaO2 = 97 mm Hg, PaCO2 = 40 mm Hg, and PaN2 = 569 mm Hg, with water vapor remaining constant at 47 mm Hg; the total arterial gas pressure is 753 mm Hg. Note that a 7-mm Hg pressure gradient exists between the alveolar gas pressure and that of the arterial blood.
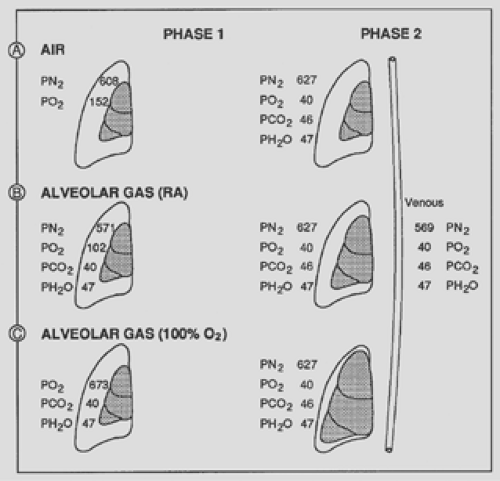 Figure 57-1. Gas partial pressures initially present in a pneumo- thorax when air entry comes from room air (A); alveoli, subject breathing room air (RA) (B); and alveoli, subject breathing 100% O2 (C). After equilibration (phase 1), the resulting gas partial pressures become identical regardless of the initial gas composition. Note that the volume change of the pneumothorax during phase 1 was least when the pneumothorax was initially constituted from room air; intermediate when from alveolar air, with the patient breathing room air; and much greater when the patient was breathing 100% O2. If a patient receives supplemental O2 for more than 2 or 3 minutes, the PN2 in the alveoli and in the blood decreases proportionally. For example, if a subject received 50% O2, the partial pressure of N2 would decrease in the alveolar air, and subsequently in the venous blood, from 569 mm Hg to approximately 350 mm Hg. Because the P[v with bar above]o2 does not increase significantly with an increase of inspired O2, the pressure gradient between a pneumothorax would be greatly increased, from 58 mm Hg to 277 mm Hg in this case.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|