Figure 30.1 Relative incidence of leiomyoma in the esophagus.
Incidence and Presentation of Leiomyoma
LMs have traditionally been thought of as very rare. Seremetis et al.9 stated that esophageal carcinoma is 50 times more common than LM. However, the incidence of LM varies greatly depending on the degree of inspection. Recent papers with detailed pathologic inspection have reported a much higher incidence of esophageal LM than previously thought. For example, in a study of 150 esophagectomy specimens, there was a 47% incidence of seedling (<7 mm) LM tumors and a 10% incidence of seedling GISTs.10 This would suggest that the incidence of LMs and GISTs is actually higher than carcinoma. This is relevant when considering the management of asymptomatic lesions 1 to 2 cm in size.
Most small esophageal submucosal tumors are asymptomatic, and are usually detected during routine upper endoscopy. When symptoms do occur, dysphagia and retrosternal discomfort are the two most common complaints.11
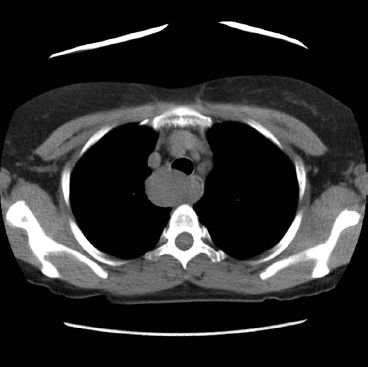
Figure 30.2 A computed tomography (CT) of a leiomyoma that presented as a proximal esophageal mass.
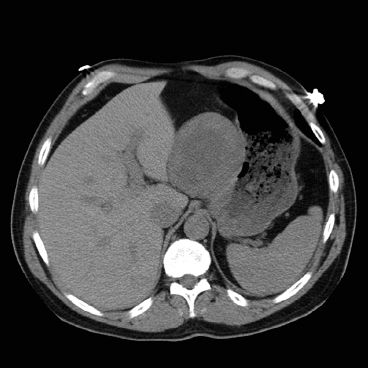
Figure 30.3 CT of a GIST that presented at the gastroesophageal junction.
 INDICATIONS/CONTRAINDICATIONS
INDICATIONS/CONTRAINDICATIONS
There is a general consensus that esophageal LMs should be surgically removed in symptomatic patients. Most authors also advocate removal of lesions >5 cm in size, lesions that are enlarging, or those in which a definite diagnosis cannot be obtained by biopsy.11 There is some controversy regarding the need for surgery in asymptomatic patients. Many surgeons believe that asymptomatic patients with small, well-encapsulated LMs <5 cm in size can be observed.12 Reports of malignant degeneration have been vanishingly rare and are probably due to misdiagnosis.7 Other surgeons advocate resection of all lesions.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
A barium esophagram may be the only diagnostic study needed for classic esophageal LMs that are symptomatic and easily resectable. If additional information is needed, an endoscopic ultrasound (EUS) is the best imaging procedure to further characterize submucosal tumors of the esophagus. The differential diagnosis of submucosal tumors can be narrowed based on the sonographic layer of origin, the echo pattern, and the margins of the lesion. LMs, GISTs, and leiomyosarcomas originate from the fourth sonographic layer (muscularis propria). LMs appear hypoechoic, well-circumscribed, and homogeneous. At the other end of the spectrum, leiomyosarcomas appear heterogeneous with irregular margins. Although LMs and leiomyosarcomas appear quite different, GISTs can have features of both and therefore, cannot be reliably distinguished by EUS.13
Indication for Biopsy
The threshold to biopsy submucosal tumors should be fairly low. Landi and Palazzo13 recommended a histologic diagnosis whenever possible. For tumors arising from the muscularis propria, <2 cm in size, and with smooth borders, the overwhelming majority will be LMs14 and a biopsy may be omitted.
For lesions 2 to 5 cm in size, the decision to biopsy is predicated on the decision to resect or not. If resection is planned, then a biopsy can be deferred. If surveillance is the planned course of action, then biopsy is recommended to rule out a GIST.
One concern of obtaining an endoscopic biopsy is that scarring between the tumor and the submucosa will lead to a higher rate of mucosal perforation during resection.15 However, more recently, surgeons have noted that fine-needle aspirations (FNAs) done prior to surgical extirpation do not lead to any significant dissection difficulties.8,16
Most authors advocate FNA if EUS shows a tumor with irregular borders or that is not confined to the muscularis propria. Because there is significant overlap in size, growth rate, and EUS appearance between benign and malignant tumors, some authors set a low threshold to obtain biopsies. For example, Blum recommends EUS–FNA for submucosal tumors larger than 2 cm, those that are enlarging on serial examination, or those with activity on PET scan.8 On the other hand, Lee et al.17 defer preoperative biopsy of well-encapsulated tumors confined to the muscularis propria up to 5 cm.
For submucosal tumors larger than 5 cm, most authors advocate preoperative biopsy. At this size, the likelihood of GIST or leiomyosarcoma is higher. Moreover, 5 cm is the size at which GISTs move into the intermediate risk category despite the mitotic count. For GISTs 5 cm or larger, the preoperative biopsy may determine the decision to enucleate versus perform an esophagectomy.
The accuracy of EUS-guided or EUS-assisted needle biopsy is based on studies analyzing gastric GISTs. The average diagnostic yield for FNA was 67%. The average diagnostic yield for fine needle biopsy or true-cut biopsy was 91%.14 Forceps biopsy should be avoided.
In our experience of 20 patients with submucosal tumors, 15 of the 20 (75%) were leiomyomas and 3 were GISTs (15%). There were 12 tumors ≤5 cm of which 2 were GISTs.18 In a large series of GISTs, leiomyomas, and leiomyosarcomas, GISTs (17/68 tumors, 25%) ranged from 2.6 to 25 cm in maximum diameter (median: 8 cm). The leiomyomas (48/68 tumors, 71%) ranged from 1 to 18 cm in maximum diameter with a median maximum diameter of 5 cm.19
 SURGERY
SURGERY
General Principles
The approach to the upper and middle esophagus is through a right thoracotomy or right video-assisted thoracoscopic surgery (VATS). The traditional approach to the lower third of the esophagus is through a left thoracotomy. With the application of minimally invasive techniques, the lower third of the esophagus can be approached from the right chest, left chest, or abdomen. The surgeon’s experience should dictate the approach to the lower esophagus rather than the laterality of the tumor. Surgeons with minimally invasive experience have approached tumors of the lower esophagus via a right VATS or left VATS.18 Laparoscopy provides excellent visualization and exposure for tumors in the distal thoracic esophagus, intra-abdominal esophagus or gastroesophageal junction. This approach permits fundoplication in the event of extensive myotomy.
Right VATS Technique
The patient is intubated with a double-lumen tube. Endoscopy is performed to confirm the location of the tumor. Knowing the precise location of the tumor is critical for tumors in the upper esophagus because access via a VATS approach can be an issue if the tumor is less than 20 cm from the incisors. Very high tumors may need to be excised via a cervical incision approach.
For mid-to-lower esophageal tumors, we place a camera port in the eighth intercostal space, at the mid-axillary line. A 5-mm port is placed at the eighth or ninth intercostal space, posterior to the posterior axillary line, for the autosonic shears (Covidien), harmonic scalpel (Ethicon), or other energy device. A 5-mm port is placed in the anterior axillary line at the fourth intercostal space to retract the lung anteriorly. The last 5-mm port is placed just posterior to the tip of the scapula and is used for retraction and countertraction by the surgeon. The surgeon stands at the back of the patient; the assistant with the camera and retractor stands at the front.
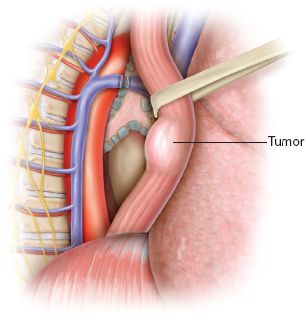
Figure 30.4 Circumferential dissection of the esophagus taking care to avoid the vagus nerves.



