INDICATIONS/CONTRAINDICATIONS
Indications
 High-grade dysplasia (HGD): Recent reports have shown superior results of RFA compared with surveillance alone, and RFA is associated with significantly less morbidity than esophagectomy.
High-grade dysplasia (HGD): Recent reports have shown superior results of RFA compared with surveillance alone, and RFA is associated with significantly less morbidity than esophagectomy.
 Low-grade dysplasia (LGD): Good success rates are reported in the literature; however, the natural course of LGD in BE is uncertain, and therefore surveillance is also an appropriate strategy.
Low-grade dysplasia (LGD): Good success rates are reported in the literature; however, the natural course of LGD in BE is uncertain, and therefore surveillance is also an appropriate strategy.
 Non-dysplastic BE: Most controversial indication for RFA because the risk of progression to cancer in these patients is small. Good results with RFA for BE have been reported in the literature.
Non-dysplastic BE: Most controversial indication for RFA because the risk of progression to cancer in these patients is small. Good results with RFA for BE have been reported in the literature.
Contraindications
 Pregnancy.
Pregnancy.
 Prior radiation therapy to the esophagus.
Prior radiation therapy to the esophagus.
 Esophageal varices.
Esophageal varices.
 Prior Heller myotomy.
Prior Heller myotomy.
 Nodular or ulcerated BE: Nodular or ulcerated areas in BE must undergo EMR to rule out carcinoma.
Nodular or ulcerated BE: Nodular or ulcerated areas in BE must undergo EMR to rule out carcinoma.
 Esophageal adenocarcinoma: Standard management for esophageal cancer remains esophagectomy. For intramucosal carcinomas, endoscopic resection followed by RFA of residual BE is becoming a viable option.
Esophageal adenocarcinoma: Standard management for esophageal cancer remains esophagectomy. For intramucosal carcinomas, endoscopic resection followed by RFA of residual BE is becoming a viable option.
 BE or dysplasia in the setting of a known stricture is a relative contraindication.
BE or dysplasia in the setting of a known stricture is a relative contraindication.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
All patients should undergo a preoperative endoscopy with biopsy and identification of the type and extent of BE and/or dysplasia. This allows for proper planning for the procedure, such as the need for multiple initial treatments for longer lesions or consideration of the presence of a hiatal hernia when selecting a sizing balloon. Patients should be maintained on proton pump inhibitor (PPI) therapy and should not eat or drink the evening prior to the procedure.
 SURGERY
SURGERY
RFA Device
In 2000, the BARRX corporation (Sunnyvale, CA) introduced the HALO 360 RFA device, which received FDA approval for clinical use in 2001 (Fig. 31.1). The HALO 360 is a pneumatic balloon that is connected to a computerized energy source. The balloon-based ablation catheter contains a microelectrode array, encircling a balloon, that is capable of delivering radiofrequency energy. The array is composed of 60 tightly spaced, bipolar electrodes that circumferentially surround the balloon and cover a length of 3 cm. The energy source calculates the optimal RFA balloon size, utilizing a computer-controlled balloon sizer (Fig. 31.2 A,B). It then distributes energy through the electrode on the RFA balloon. Ten to twelve J/cm2 are delivered in a 360-degree radius over the length of the 3-cm electrode allowing for a treatment depth of 1,000 microns. This depth is critical to ensure complete treatment of the BE, which has been measured at 500 microns, while avoiding deeper tissue destruction, which could lead to perforation or strictures.6 A second RFA catheter, the HALO 90, was introduced by BARRX in 2006. This catheter is affixed to the end of an endoscope and delivers the same amount of energy as the HALO 360 but confined to a 90-degree radius. This allows for treatment of non-circumferential BE without treating adjacent, normal, esophageal mucosa (Fig. 31.3).

Figure 31.1 HALO 360 RFA catheter. Image supplied by BARRX Corporation, Sunnyvale CA, with permission.
RFA Procedure
Ablation procedures may be performed under conscious sedation or general anesthesia in an outpatient setting. The procedure begins with an esophagoscopy to carefully identify the location and length of the IM or dysplasia. N-acetylcysteine (1%) is then injected through the endoscope to remove mucus from the mucosa to improve the contact of the electrodes with the tissue. A stiff guidewire (e.g., Amplatz extra stiff 0.035-inch; Cook Europe, Bjaeverskov, Denmark) is then placed through the endoscope into the stomach and the scope is removed. A sizing catheter, with a 4-cm long balloon at its distal end, is placed over the guidewire to measure the diameter of the esophagus. This is performed as a blind procedure using the 1-cm scale on the catheter shaft for reference, and measurements are begun with the catheter positioned 5 cm above the proximal extent of the IM. The sizing balloon is inflated by the HALO 360 energy generator, and the mean esophageal diameter is automatically calculated for the entire length of the 4-cm balloon. Measurement is then repeated for every centimeter of the targeted portion of the esophagus. Once this has been done, an appropriately sized RFA balloon can be chosen (22, 25, 28, 31, or 34 mm). The balloon may oversize the esophagus in patients with large hiatal hernias, and in these patients, the operator should choose the average or smaller balloon size to prevent esophageal injury.

Figure 31.2 A: HALO energy generator. B: Sizing balloon. Images supplied by BARRX Corporation, Sunnyvale CA, with permission.

Figure 31.3 HALO 90 catheter affixed to an endoscope. Image supplied by BARRX Corporation, Sunnyvale CA, with permission.
The RFA catheter is then placed over the guidewire, positioned at the level of the abnormal mucosa and the endoscope is placed down the esophagus next to the RFA catheter (Fig. 31.4). The RFA balloon is inflated and energy is delivered by stepping on a foot pedal control, while simultaneously, suction is applied on the endoscope to ensure good contact between the IM/dysplasia and the electrode. The electrode is advanced in 3 cm increments until the entire length of the targeted tissue has been ablated (Fig. 31.5). The RFA catheter is then removed and cleaned to clear the sloughed mucosa off of the electrode. A soft plastic cap is attached to the endoscope and used to debride all coagulative debris away from the treated area to prepare the area for a second treatment. A second treatment is then performed. For ablation of IM, 10 J/cm2 is used, and 12 J/cm2 is used for ablation of dysplastic tissue. The length of treatment for a single session should be limited to 6 cm to decrease the chance of stricture formation.
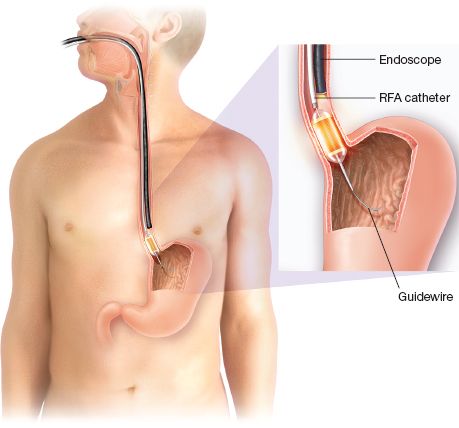
Figure 31.4 Circumferential ablation with balloon-based RFA system.
At a minimum of 8 weeks after the first circumferential ablation, patients are rescheduled for a second ablation. Localized IM found on subsequent surveillance endoscopy can be treated with the HALO 90 catheter. Focal ablation, delivered with the HALO 90 system, is performed by placing the endoscope-mounted electrode in contact with the target epithelium by deflecting the tip of the endoscope upward, flatly opposing the electrode to the esophageal wall (Fig. 31.6). Energy is delivered twice to the ablation target. After the first application, the endoscope is removed, the device is cleaned, and the procedure is repeated. This results in a total of four treatments to each area. Ablation can be repeated every 2 to 3 months until all IM has been eradicated visually and eradication confirmed histologically.
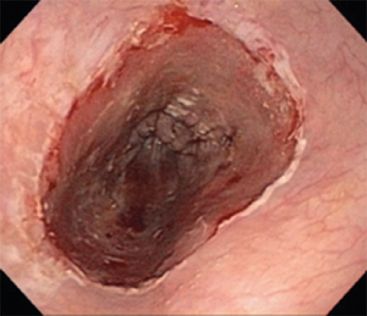
Figure 31.5 Esophagoscopy revealing the ablation zone.
 POSTOPERATIVE MANAGEMENT
POSTOPERATIVE MANAGEMENT
Almost all patients experience some chest discomfort, sore throat, difficulty or pain with swallowing, and/or nausea after therapy. This may be treated with viscous lidocaine, liquid acetaminophen with or without codeine, and antiemetics. Antisecretory therapy with PPIs is continued before and after the procedure to minimize discomfort and allow the esophagus to heal and regenerate with squamous epithelium. Patients are placed on a liquid diet for 24 hours after the procedure and may then gradually advance their diet as tolerated. In absence of long-term follow-up data for RFA, it is advised that patients undergo endoscopy 2 and 6 months after the last treatment and then annually.
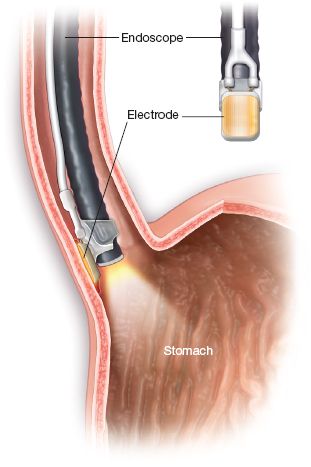
Figure 31.6 Focal ablation with endoscope-mounted HALO 90 RFA system.



