Chapter 145
Renovascular Disease
Open Surgical Treatment
Justin B. Hurie, Kimberley J. Hansen
Based on a chapter in the seventh edition by Christopher J. Godshall and Kimberley J. Hansen
Following Goldblatt’s innovative work defining the causal relationship between renovascular disease and hypertension in 1934,1 the surgical management of renovascular disease has subsequently evolved through three eras—nephrectomy, open surgical correction, and finally renal artery angioplasty with or without stenting (Fig. 145-1). Each era was met with initial enthusiasm only to be tempered by the modest results of each treatment. Nephrectomy is now rarely indicated in the management of renovascular disease and the benefits of renal artery stenting have recently been called into question, so we await the results of ongoing trials for definitive guidance (see Chapter 146). In this chapter, we review the evolution in patient selection for open repair of renovascular disease and then describe specific surgical approaches and their expected outcomes in contemporary practice.
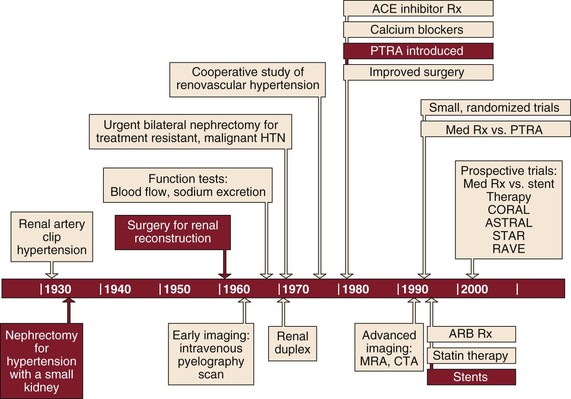
Figure 145-1 Timeline showing evolution of the surgical management of renovascular disease through three eras. ACE, Angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CTA, computed tomographic angiography; HTN, hypertension; MRA, magnetic resonance angiography; PTRA, percutaneous transluminal renal angioplasty; Rx, therapy.
Indications for Surgical Repair
The rationale for treatment of renovascular disease by any method is to improve event-free survival. In the past, hypertension response has been the primary outcome measure for most of the interventions for renovascular disease. However, our experience in 500 consecutive patients treated for atherosclerotic renovascular disease demonstrated a lack of association between improved blood pressure response and cardiovascular morbidity or mortality.2 Instead, severe preoperative hypertension was independently associated with improved dialysis-free survival. This observation suggests a possible shift in the evaluation and management of atherosclerotic renal artery disease.3 Severe hypertension could be viewed as a key preoperative characteristic favoring clinical benefit, whereas renal function after operation could be considered the key response. Early incremental increase in excretory renal function has been the primary determinant of dialysis-free survival for all atherosclerotic patients.2,4
Unfortunately, once renovascular disease is identified in combination with severe hypertension or excretory renal insufficiency, discriminating predictors of blood pressure and renal function response are lacking. Lateralizing renin assays have predicted blood pressure benefit but not blood pressure cure.5,6 Moreover, renal function response in patients with ischemic nephropathy is uncertain after renal artery intervention.4 Studies have also described significant correlation between parameters derived from segmental renal artery Doppler spectral analysis (i.e., resistive index) and response to intervention as well as progression to dialysis dependence.7,8 However, we have not been able to reproduce these results for open operative management of renal artery disease.9 Rather, in our clinical experience with more than 800 patients with atherosclerotic renal artery disease submitted to open operative repair, factors favoring recovery of renal function after operation have included severe preoperative hypertension, bilateral or global atherosclerotic renovascular disease due to high-grade (>90%) stenosis or renal artery occlusion, and rapidly deteriorating renal function before surgery.4,6,10–12 When each of these favorable features was expressed in patients considered permanently dialysis dependent, 70% of patients submitted to open operative repair were permanently removed from dialysis.4,13 Given the relationship of renal function to both quality and quantity of life, predictors of function response after renal artery intervention should be a primary focus for future investigation.
The quality of studies designed to define the natural history of the atherosclerotic renal artery has varied widely.10,14–23 Most commonly, authors have considered anatomic progression of atherosclerotic renovascular disease a certainty, one associated with an inevitable decline in kidney size and kidney function. This view has been cited to support intervention for renal artery disease whenever it is discovered, even in the absence of hypertension or renal insufficiency.
On the basis of a recent prospective, population-based study of progression of atherosclerotic renovascular disease in the elderly,24 we do not favor “prophylactic” intervention for asymptomatic renovascular disease. This longitudinal cohort study included 119 Cardiovascular Health Study participants, with 235 kidneys for study, and no kidney progressed to an occlusion during a mean follow-up of 8.5 years. In the Cardiovascular Health Study participants with mild to moderate hypertension, progression to hemodynamically significant renal artery stenosis was observed in only 4%, an annualized rate of 0.5% per year.24
Although we do not recommend prophylactic renal revascularization in the absence of hypertension or renal insufficiency, empirical renal artery repair is appropriate under select circumstances. The term empirical repair implies that hypertension, excretory renal dysfunction, or both are present, although a causal relationship between the renal artery lesion and these clinical sequelae has not been established. Open repair of a unilateral renal artery lesion may be appropriate as an independent procedure when the results of functional studies are negative or when functional studies have not been performed, when hypertension remains severe and uncontrollable despite maximal drug therapy, and when the patient is relatively young without significant risk factors for operation.25 Empirical unilateral repair in patients with ischemic nephropathy has been associated with a small proportion of function improvement and cannot be recommended as a clinically proven therapeutic intervention.4,13
When a patient has bilateral renal artery stenoses and hypertension, the decision for empirical open renal reconstruction is based on the severity of hypertension and the severity of the renal artery lesions. When disease consists of severe stenosis of one renal artery and only mild to moderate contralateral disease, it is treated as a unilateral lesion. If both renal artery lesions are moderately severe (60% to 80% diameter-reducing stenosis), revascularization is undertaken only if associated hypertension is severe. In contrast, if both renal artery lesions are severe (>80% stenosis) and the patient has severe hypertension, bilateral simultaneous renal revascularization is performed, especially when excretory renal insufficiency is present. However, because the degree of azotemia usually parallels the severity of hypertension, a patient who presents with severe excretory renal insufficiency but only mild hypertension usually has concomitant renal parenchymal disease from another cause (e.g., diabetes). Characteristically, renovascular disease contributing to severe azotemia or dialysis dependence is associated with severe hypertension and severe bilateral stenoses or renal artery occlusions.2,4,10,13
Open Operative Strategy
The presence of hypertension is considered a prerequisite for renal artery intervention and functional studies are used to guide the management of unilateral lesions. Empirical renal artery repair is performed without functional studies when hypertension is severe, renal artery disease is bilateral, or the patient has ischemic nephropathy.2,6,26 Prophylactic renal artery repair in the absence of hypertension, whether as an isolated procedure or in combination with aortic reconstruction, is not recommended. During surgical reconstruction, all hemodynamically significant renal artery disease is corrected in a single operation, with the exception of disease requiring bilateral ex vivo reconstructions, which are staged. Nephrectomy is reserved for a nonfunctioning kidney (i.e., ≤10% function by renography) with unreconstructable renal artery disease.4,10,18 Direct aortorenal reconstructions are preferred to indirect methods because concomitant disease of the celiac axis is present in 40% to 50% of patients and bilateral repair is required in half.4,6 Failed renal artery repair is also associated with a significantly increased risk of dialysis dependence.26 To minimize these failures, intraoperative duplex ultrasonography is used to evaluate the technical results of surgical repair before closure.27
The question of optimal management of renovascular disease contributing to hypertension or renal insufficiency is unanswerable. In the absence of level I data, advocates of each form of management (e.g., medical, surgical, angioplasty) cite selective clinical data to support their particular view.28
Our experience with percutaneous management as a substitute for open operative repair has demonstrated only modest hypertension and renal function response.29,30 Consequently, we continue to advocate open operative repair for select patients. These include children with hypoplastic lesions,31 adults with dysplastic lesions other than medial fibroplasia, and patients with medial fibroplasia involving branch and segmental renal arteries complicated by renal artery aneurysm.32 We advocate open operative repair for atherosclerotic renovascular disease in good-risk patients younger than 65 years who demonstrate global renal ischemia manifested by critical stenosis and renal artery occlusion, especially when severe hypertension is associated with excretory renal insufficiency with rapidly deteriorating excretory function.2,4,13 Selection criteria for repair of renal artery aneurysms are discussed in Chapter 148, but we have continued to favor open operative repair as opposed to coiling, stenting, or stent-grafting of these lesions.32
Management Options
Preoperative Preparation
Antihypertensive medications are reduced during the preoperative period to the minimum necessary for blood pressure control. Frequently, patients requiring large doses of multiple medications for hypertension management will have requirements reduced while hospitalized at bed rest. If continued therapy is required, vasodilators (e.g., amlodipine) and selective β-adrenergic blocking agents (i.e., atenolol, metoprolol) are useful. There are few adverse effects on hemodynamics when these agents are combined with general anesthesia. If an adult’s diastolic blood pressure exceeds 120 mm Hg, the operative treatment should be postponed until the pressure is brought under control. In this instance, the combination of intravenous nicardipine and esmolol is administered in an intensive care setting with continuous intra-arterial blood pressure monitoring. Similarly, if the patient has significant heart disease, pulmonary artery wedge pressure and cardiac index are monitored to maintain optimal cardiac performance before and after operation.
Certain measures are used in almost all open renal artery operations. Mannitol is administered intravenously in 12.5-g doses early in the operation during aortic and renal artery dissection. Repeated doses are administered before and after periods of renal ischemia, up to a total dose of 1 g per kilogram of patient body weight. Just before renal artery cross-clamping, 100 units of heparin per kilogram of body weight are given intravenously, and systemic anticoagulation is verified by activated clotting time. Unless it is required for hemostasis, protamine is not routinely administered for reversal of heparin at the completion of the operation.
Mobilization and Dissection
Midline Exposure
A midline abdominal incision is made for operative repair of atherosclerotic renal artery disease when bilateral renal artery repair or combined aortorenal repair is planned. The patient is positioned supine with the umbilicus at the level of the table break, with the operating table flexed 10 to 15 degrees. To obtain full exposure of the upper abdominal aorta and renal branches, it is important that the last 1 or 2 cm of the proximal incision be made coursing to one side of the xiphoid (Fig. 145-2A). Some type of fixed mechanical retraction is also advantageous, particularly when combined aortorenal procedures are required. Otherwise, extended flank and subcostal incisions are reserved for correction of unilateral branch renal artery lesions or splanchnorenal bypass.
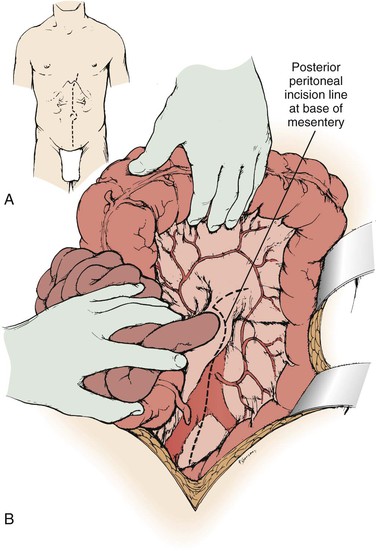
Figure 145-2 A and B, Exposure of the aorta and left renal hilum through the base of the mesentery. Extension of the posterior peritoneal incision to the left, along the inferior border of the pancreas, provides entry to an avascular plane posterior to the pancreas. This allows excellent exposure of the entire left renal vein and hilum as well as of the proximal right renal artery. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
When the midline xiphoid to pubis incision is used, the posterior peritoneum overlying the aorta is incised longitudinally and the duodenum is mobilized at the ligament of Treitz (Fig. 145-2B). During this maneuver, it is important to identify and to spare the meandering mesenteric vessel that courses at this level. Finally, the duodenum is reflected to the patient’s right to expose the left renal vein. By extending the posterior peritoneal incision to the left along the inferior border of the pancreas, an avascular plane posterior to the pancreas can be entered (Fig. 145-2B) to expose the entire left renal hilum. This exposure is of special importance when there are distal renal artery lesions to be managed (Fig. 145-3A). The left renal artery lies posterior to the left renal vein. In some cases, the vein can be retracted cephalad to expose the artery; in other cases, caudal retraction of the vein provides better access. Usually, the gonadal and adrenal veins, which enter the left renal vein, must be ligated and divided to facilitate exposure of the distal artery. Frequently, a lumbar vein enters the posterior wall of the left renal vein, and it can be injured easily unless special care is taken (Fig. 145-3B). The proximal portion of the right renal artery can be exposed through the base of the mesentery by retracting the left renal vein cephalad and the vena cava to the patient’s right (Fig. 145-3C). However, the distal portion of the right renal artery is best exposed by mobilizing the duodenum and right colon medially; the right renal vein is mobilized and usually retracted cephalad to expose the artery.
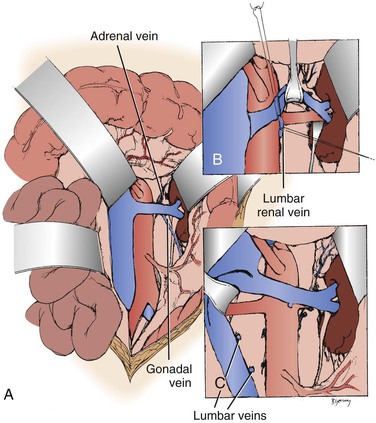
Figure 145-3 A, Exposure of the proximal right renal artery through the base of the mesentery. B, Mobilization of the left renal vein by ligation and division of the adrenal, gonadal, and lumbar-renal veins allows exposure of the entire left renal artery to the hilum. C, On occasion, lumbar veins are ligated and divided to allow retraction of the vena cava to the right. Often, adequate exposure of the proximal renal artery disease can be obtained without this maneuver. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
Flank Exposure
When a branch renal artery repair is required, especially when ex vivo technique is used or when the supraceliac aorta is used as an inflow source for aortorenal bypass, an extended flank incision is useful. With the ipsilateral flank bumped, the incision extends from the opposite semilunar line into the flank, bisecting the abdominal wall between the costal margin and iliac crest. A left or right visceral mobilization allows access to the renal vasculature and the aortic crus. The crus can be divided, and an extrapleural dissection of the descending thoracic aorta can provide access to the T9-10 thoracic aorta for proximal control and anastomosis.33,34
Whether right or left branch renal artery exposure is required, the key is creation of the correct dissection plane between the mesentery anteriorly and Gerota’s fascia posteriorly. The renal vein is identified first and mobilized from caval origin to renal hilum. On the right, small venous branches at the junction with the vena cava require ligation. The adrenal, gonadal, and lumbar branches on the left may be sacrificed to facilitate exposure.
Branch renal artery exposure on the right is achieved by colonic and duodenal mobilization. First, the hepatic flexure is mobilized at the peritoneal reflection (Fig. 145-4). With the right colon retracted medially and inferiorly, a Kocher maneuver mobilizes the duodenum and pancreatic head to expose the inferior vena cava and right renal vein (Fig. 145-5). Typically, the right renal artery is located just inferior to the accompanying vein, which can be retracted superiorly to provide the best exposure. Although accessory vessels may arise from the aorta or iliac vessels at any level, all arterial branches coursing anterior to the vena cava should be considered accessory right renal branches and carefully preserved (Fig. 145-6).
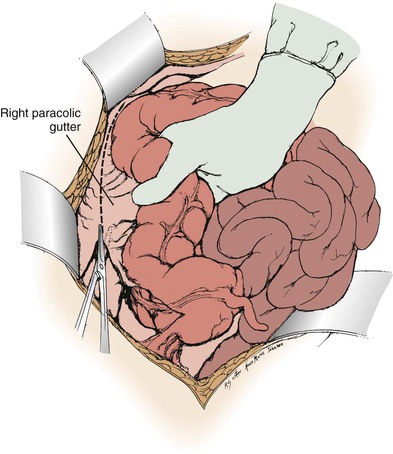
Figure 145-4 Exposure of the distal right renal artery begins with mobilization of the ascending colon and hepatic flexure. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
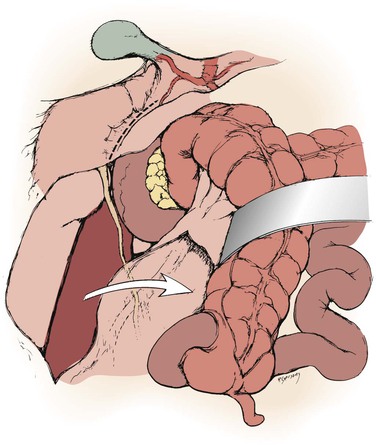
Figure 145-5 With the right colon mobilized medially, a Kocher maneuver exposes the right renal hilum. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
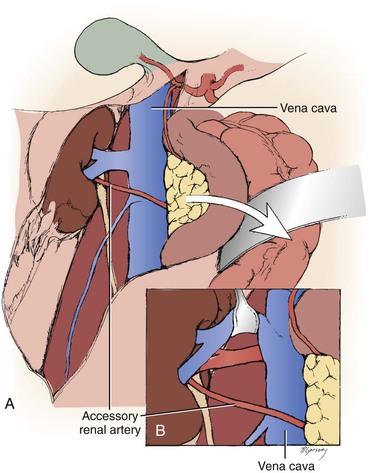
Figure 145-6 A, Arteries encountered anterior to the vena cava should be considered accessory renal arteries and preserved. B, The right renal vein is typically mobilized superiorly for exposure of the distal right renal artery. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
When bilateral renal artery lesions are to be corrected and when correction of a right renal artery lesion or bilateral lesions is combined with aortic reconstruction, these exposure techniques can be modified. Extended aortic exposure may be provided by mobilizing the base of the small bowel mesentery to allow complete evisceration of the entire small bowel, right colon, and transverse colon. For this extended exposure, the posterior peritoneal incision begins with division of the ligament of Treitz and proceeds along the base of the mesentery to the cecum and then along the lateral gutter to the foramen of Winslow (Fig. 145-7A). The inferior border of the pancreas is fully mobilized to enter a retropancreatic plane, thereby exposing the aorta to a point above the superior mesenteric artery. Through this modified exposure, simultaneous bilateral renal endarterectomies, aortorenal grafting, or renal artery attachment to the aortic graft can be performed with complete visualization of the entire aorta and its branches. Another useful technique for suprarenal aortic exposure is to partially divide both diaphragmatic crura as they pass behind the renal arteries to their paravertebral attachment. This partial division of the crura allows the aorta above the superior mesenteric artery to be easily visualized and mobilized for suprarenal cross-clamping (Fig. 145-7B).
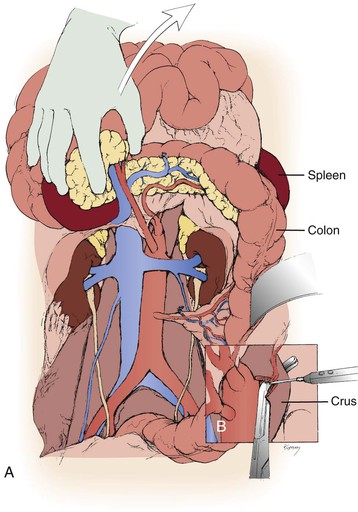
Figure 145-7 A, For bilateral renal artery reconstruction combined with aortic repair, extended exposure can be obtained with mobilization of the cecum and ascending colon. The entire small bowel and right colon are then mobilized to the right upper quadrant and retracted superiorly. B, Partial division of the diaphragmatic crura exposes the origin of the mesenteric vessels. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
Direct Surgical Techniques
A variety of open operative techniques have been used to correct renal artery disease, but three basic operations have been most frequently used: aortorenal bypass, renal artery thromboendarterectomy, and renal artery reimplantation. No single approach provides optimal repair for all types of renovascular lesions in all patients. Aortorenal bypass using saphenous vein is probably the most versatile technique; however, transaortic thromboendarterectomy is especially useful for orificial atherosclerosis involving multiple renal arteries. On occasion, the renal artery will demonstrate sufficient redundancy to allow reimplantation, probably the simplest technique and one that is particularly appropriate to hypoplastic renal artery lesions in children.30,31
Aortorenal Bypass
Three types of materials are available for aortorenal bypass: autologous saphenous vein, autologous hypogastric artery, and synthetic graft. The decision as to which graft should be used depends on a number of factors. In most instances, the authors prefer the saphenous vein for older adults. However, if the vein is small (<4 mm in diameter) or sclerotic, the hypogastric artery or a synthetic graft may be preferable. In addition, vein enlargement can be anticipated in virtually all young adults. Although structural immaturity has been cited31 for enlargement and aneurysmal degeneration when saphenous vein is used for renal artery reconstruction in children, the normal renal blood flow in the young resembles an arteriovenous fistula with continuous forward flow. This fact alone may account for vein enlargement in the young patient with normal renovascular resistance. In lieu of vein, a 6-mm thin-wall polytetrafluoroethylene (PTFE) graft is satisfactory when the distal renal artery is of sufficient caliber (≥4 mm). Hypogastric artery autograft is preferred for aortorenal bypass in children when reimplantation is not possible.28,31,35,36
End-to-side distal anastomoses are occasionally used to include polar renal arteries. In this instance, end-to-side anastomosis between the polar renal artery and the graft is performed after the proximal aortic anastomosis. After the end-to-side anastomosis is completed, the most distal main renal artery reconstruction is made in an end-to-end fashion (Fig. 145-8).
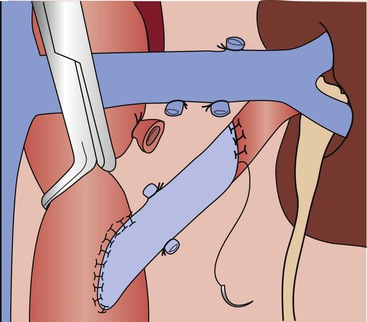
Figure 145-8 Technique for end-to-end aortorenal bypass grafting. The length of arteriotomy is at least three times the diameter of the artery to prevent recurrent anastomotic stenosis. For the anastomosis, 6-0 or 7-0 monofilament polypropylene sutures are used in continuous fashion with loupe magnification. If the apex sutures are placed too deeply or with excess advancement, stenosis can be created, posing a risk of late graft thrombosis. (From Benjamin ME, et al: Techniques in renal artery reconstruction: part I. Ann Vasc Surg 10:306-314, 1996.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


