Chapter 147
Renovascular Disease
Fibrodysplasia
Bengt Lindblad, Anders Gottsäter
Fibromuscular dysplasia (FMD, also known as arterial fibrodysplasia) is a nonatherosclerotic, noninflammatory angiopathy of unknown cause. It affects medium-sized arteries—most commonly the renal arteries—and can cause renovascular hypertension. FMD of the renal arteries was first described in 1938 by Leadbetter and Burkland1 in a 5-year-old boy. Involvement of other arteries was subsequently recognized, and carotid FMD was reported by Palubinskas and Ripley2 in 1964.
Epidemiology
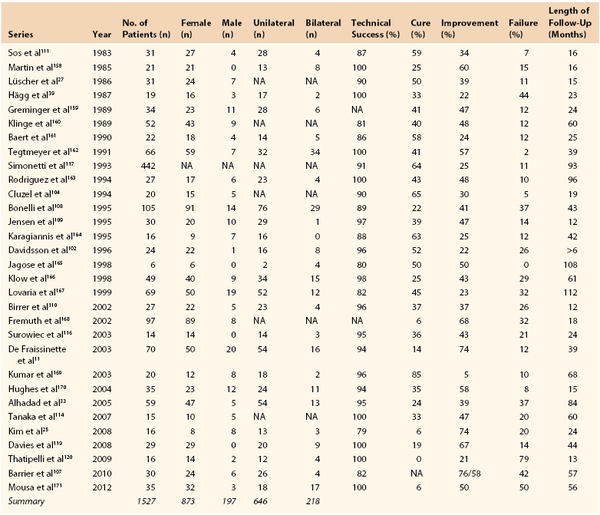
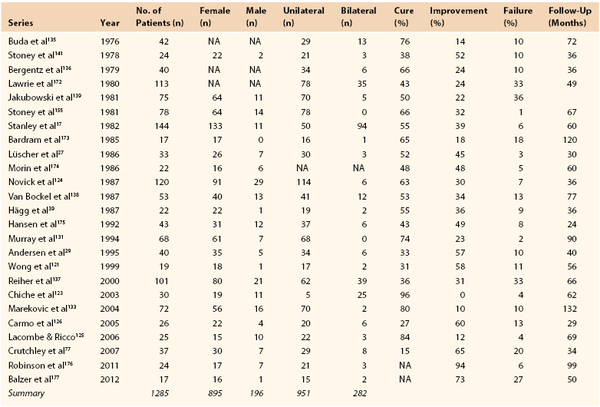
Symptomatic fibrodysplastic renal artery stenosis reportedly occurs in 0.4% of the population,3 but the prevalence of asymptomatic FMD in potential renal donors is around 4%.4–6 The true prevalence is difficult to ascertain because there are no easily applicable screening tests. FMD is most often diagnosed in patients aged 15 to 70 years7,8 but it has been reported from infancy9,10 to age 89 years.11–13 The medial type of FMD is most common (68%-91%)8,14 and is more than four times more prevalent in females than in males.3,4,8,14 In contrast, the less common intimal type is more prevalent in men.3,15 In the studies summarized in Tables 147-1 and 147-2, 80% to 82% of patients in both endovascular and open surgery series were female, for a male-to-female ratio of 1 : 4.5. Lesions are bilateral in about 25% of cases (see Tables 147-1 and 147-2). In the case of bilateral lesions, it was not always possible to ascertain whether both lesions required treatment.16 Stanley et al17 reported bilateral lesions in 65% of patients; however, in only 15% of cases did this bilaterality significantly influence the management of patients. Among 337 renal FMD cases, Savard et al18 found bilateral disease in 55%, and in unilateral disease, the right renal artery was affected three times more commonly than the left. In series that reported treated sides in unilateral disease (see Tables 147-1 and 147-2), the right renal artery was affected in 66% of 503 open surgery cases and in 81% of 181 endovascular therapy cases.
FMD is more likely to be manifest in patients with treatment-resistant or malignant hypertension19,20 than in the general hypertensive population and accounts for up to 10% of all cases of renovascular hypertension21,22; the remainder of renovascular hypertension cases are caused mainly by atherosclerosis. Compared with patients with atherosclerotic renal artery stenosis, patients with FMD are younger and have both fewer risk factors for atherosclerosis and a lower occurrence of atherosclerosis in other vessels.
FMD is diagnosed most often in whites and is reported less frequently in Hispanic and Asian populations. Few reports have been published from Africa and South America.9,23–26 In the United States Registry for Fibromuscular Dysplasia, which contains data for 447 patients, 95% are white, and only 2% African Americans and 1% Hispanic and Asian individuals are registered.8 Diagnostic criteria vary and prevalence data are often derived from selected cohorts or autopsy studies, so the prevalence of FMD might be overestimated because of the difficulty of estimating the clinical significance of a morphologic stenosis.
Other vessels, such as the carotid and coronary arteries, can also be affected (Table 147-3),8 and FMD should be excluded in young people presenting with acute carotid artery dissection or occlusion (see Chapter 102). Multiple-organ involvement with FMD and cerebral or cardiac ischemia may be associated with an increased risk of complications and death.28 Multiple arterial involvements have been reported in 8 of 34 and 9 of 102 renovascular patients with FMD in earlier series,29,30 and in the registry report, 2 vascular beds were affected in 35% and 3 in 22%.8 This new information reinforces the need for a good clinical examination and at least a carotid artery investigation if FMD is found in other vascular beds.8
Table 147-3
Arterial Involvement in Fibromuscular Dysplasia Based on the United States Registry for Fibromuscular Dysplasia
| Arteries Involved | No. of Investigated Arteries in the U.S. Registry* | Frequency of Involvement (%)† |
| Total number in U.S. Registry | 447 | |
| Renal arteries | 369 | 80 (75-89) |
| Bilateral renal arteries | (23-65) | |
| Unilateral renal artery—localization: | ||
| Right renal artery | (66-81) | |
| Left renal artery | (19-34) | |
| Other arteries: | ||
| Carotid artery | 338 | 74 (3-74) |
| Vertebral artery | 224 | 37 |
| Aorta | 145 | 0 |
| Lower extremity arteries | 70 | 60 |
| Mesenteric arteries | 198 | 26 |
| Coronary arteries | 447 | 7 |
| Upper extremity arteries | 63 | 16 |
| Intracranial carotid arteries | 206 | 17 |
| Multiple vascular involvement | 35 (8-35) |
* Data from Olin JW, et al: The United States registry for fibromuscular dysplasia: results in the first 447 patients. Circulation 2012; 125:3182-3190.
† Data shown in parentheses is based on results presented in studies listed in Tables 147-1 and 147-2.
In childhood, renovascular hypertension is a more important cause of hypertension. It is found in 8% to 10% of all hypertensive children and in up to 25% of those with secondary hypertension. The causes of pediatric renal artery stenosis differ in different populations.9,23,31 FMD is the most common cause of pediatric renovascular hypertension in North America and Western Europe, whereas Takayasu’s arteritis dominates in Asia and Africa.10 Because the number of pediatric patients with FMD is small, evaluation and treatment should be centralized.
Pathogenesis
Etiology
The fact that FMD is more common among women suggests that hormonal factors may be important.52,32 Nine of the 57 women in one study, or 16%, had a previous diagnosis of hypertension during pregnancy, compared with 4% to 5% of pregnancies affected by hypertension in the general population.33 The number of pregnancies and the frequency of oral contraceptive use or hormone therapy did not differ between patients with FMD and the general population, however.8,32,34
Vessel wall ischemia may also be important for the development of FMD.35 The vasa vasorum of muscular arteries, which supply oxygen and nutrients to the arterial wall, originate from branch points of the parent arteries. Occlusion of the vasa vasorum induces the formation of dysplastic lesions in animal studies.36 The vessels most commonly affected by FMD, such as the renal, internal carotid, and vertebral arteries, have long segments that lack branches and thus have few vasa vasorum. Mechanically these arteries are subjected to repeated stretching during motion and respiration, which may injure the sparse vasa vasorum, causing arterial wall ischemia and the subsequent development of FMD. This hypothesis is supported by the observation that FMD is more common in the right renal artery,30 which is longer than the left. The longer length makes the right kidney more subject to renal ptosis,37 which is also common among patients with renal FMD.38 Vasospasm in the vessel wall might also induce ischemia in the vasa vasorum, and cases of FMD combined with Raynaud’s disease have been reported.39 However, these theories do not explain the gender difference.
FMD is associated with cigarette smoking.34 The prevalence of smoking is higher in patients with FMD than in matched controls,32,34,40 and patients with FMD who smoke have more severe arterial disease than nonsmokers.41 In the U.S. Registry for FMD, 37% of patients were current or former smokers compared with 18% reported for U.S. women.8 The mechanisms by which smoking contributes to FMD have not been elucidated, however.
The occurrence of renal FMD in siblings and identical twins suggests the possible inheritance of the disease.42 Rushton43 suggested that FMD is transmitted in an autosomal dominant manner, with incomplete penetrance and variable clinical symptoms. A French study of renal FMD showed that 11% of patients had at least one sibling with renal FMD.15 The U.S. Registry study reported a 7% incidence in relatives8; however, they also reported that stroke (54%), aneurysm (24%), and sudden death (20%) were common in first- or second-degree relatives. The presence of FMD can be easily overlooked in relatives because it may be associated with only mild hypertension or even normotension. Subclinical dysplasia of the carotid artery also occurs in patients with renal FMD,44 in accordance with a possible autosomal dominant transmission.45
Associations with polymorphisms in the angiotensin-converting enzyme (ACE) insertion allele ACE-I have been reported,46 and an autoimmune origin of FMD has been suggested by genetic associations with HLA-Drw6.34 Currently several groups are trying to delineate further gene patterns predisposing for FMD development.47
FMD might coexist with other diseases of the vessel wall and endocrine system. Ehlers-Danlos syndrome type IV has been associated with medial fibroplasias48 and should be suspected in patients with multiple aneurysms and FMD. FMD has also been reported in association with pheochromocytoma,49 Marfan’s syndrome,50 Alport’s syndrome,51 and Takayasu’s arteritis.52
Differential Diagnosis
Important differential diagnoses for FMD are type 1 neurofibromatosis,53,54 vascular Ehlers-Danlos and Williams syndrome, and vasculitis. The diagnosis of these conditions relies on associated phenotypic traits: characteristic skin lesions in type 1 neurofibromatosis55; acrogeric dysmorphism, skin elasticity, and distal joint laxity in vascular Ehlers-Danlos syndrome56; and facial dysmorphism, supra-aortic stenosis, and particular behavior in Williams syndrome.57 Genetic tests can also be used to rule out these conditions as alternative diagnoses.
Because FMD is, by definition, a noninflammatory process, it is not associated with anemia, thrombocytopenia, or the increased acute phase reactants that often occur in patients with vasculitis. Large-vessel vasculitis sometimes occurs in the absence of changes in acute phase reactants, however.58 Therefore, it might be difficult to distinguish FMD from inflammatory vessel disease in the absence of tissue samples and without laboratory markers confirming inflammation.
Pathology
In vitro studies have demonstrated increased production of collagen, hyaluronan, and chondroitin sulfate in arteries exposed to cyclic stretching.59 Mural ischemia due to functional defects in the vasa vasorum, possibly in association with developmental renal malposition, has also been postulated as a cause of FMD.60
FMD has not been found in other species except for domestic turkeys; one study reported the disease in 24 of 31 examined turkeys, with no sex differences.61
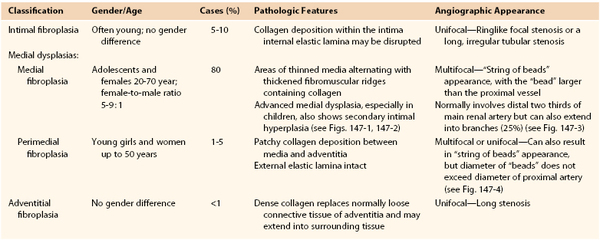
Classification
Histologically, three main types of dysplasias have been identified and are classified according to the dominant arterial wall layer involved: intimal, medial, or adventitial (Table 147-4, Figs. 147-1 to 147-4).30,62–65 It is important to distinguish among the different types of FMD because they warrant special consideration for optimal treatment when the renal artery is affected. Complications of arterial dysplasia, such as aneurysm formation (in 17% of patients with FMD, one third in the renal artery) and dissection (in 20% of patients with FMD, more common in carotid and vertebral arteries, and renal artery accounts for one fifth),8 should be classified as secondary events and differentiated from primary dysplastic lesions. Currently few dysplasias are histologically examined, and an angiographic diagnostic classification based on differentiation between unifocal and multifocal appearance on magnetic resonance (MR) imaging, computed tomography (CT), or angiography has been suggested.18 Unifocal FMD had less female predomination, is diagnosed in younger individuals, and is treated with better short- and long-term results than multifocal FMD.18 This classification has not been applied to FMD in children.
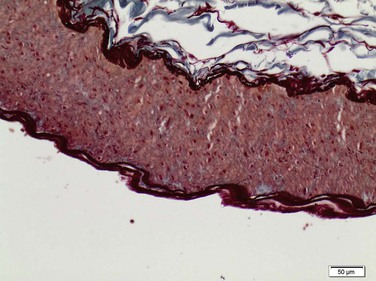
Figure 147-1 Normal renal artery with distinct wall layers. (Courtesey Malina J: Department of Pathology, Malmö, Sweden.)
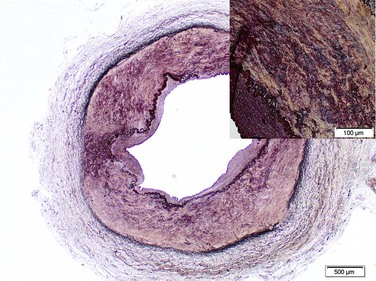
Figure 147-2 Medial fibrodysplasia with dense fibrous connective tissue in the outer media, disordered inner medial smooth muscle. (Courtesey Malina J: Department of Pathology, Malmö, Sweden.)
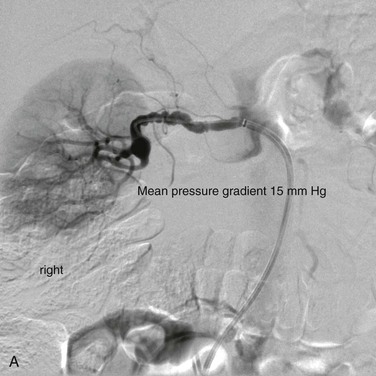
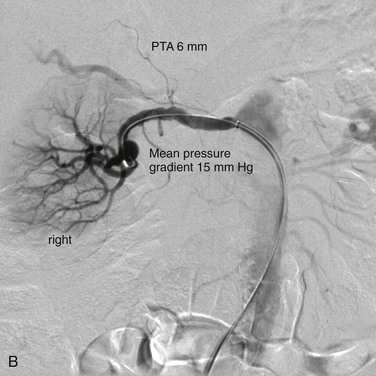
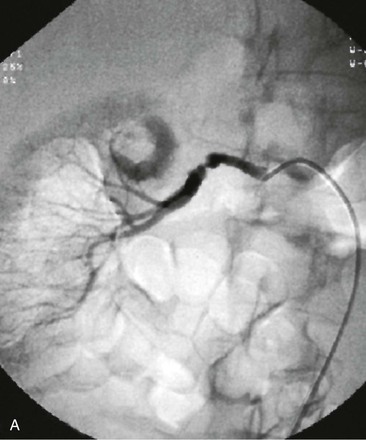
Figure 147-3 Typical selective angiographic multifocal appearance of medial fibrodysplasia with the “string of beads” in the distal main artery before (A) and after (B) percutaneous transluminal angioplasty dilatation (PTA).
Pathophysiology
Multiple septa in the renal arteries may together induce a significant reduction of renal perfusion in patients with FMD, resulting in renovascular hypertension, but the degree of renal artery stenosis is often hard to evaluate from imaging.
Subsequent reduction of arterial perfusion pressure in the stenosed kidney leads to activation of the renin-angiotensin-aldosterone (RAA) system, resulting in volume expansion and hypertension. Several mechanisms, including increased endothelin-1 (ET-1) production, local renin-angiotensin-aldosterone activation, arterial wall remodeling, and oxidative stress, help sustain the hypertension,66 which now depends not only on the renin-angiotensin-aldosterone system but also on local vasoconstrictive proliferative effects in the arterial wall, gradually leading to resistance to therapy.67 Inflammatory mediators such as high-sensitivity C-reactive protein, tumor necrosis factor-α, interleukin-6, and neopterin, and vasoconstrictive mediators such as endothelin-1 are increased in patients with renovascular hypertension.68 A separate analysis of patients with FMD, however, showed that neopterin and endothelin-1 were lower in patients with renovascular hypertension due to FMD than in those with renal artery stenosis of atherosclerotic origin.68 This finding suggests that inflammatory activation might be less important for the pathophysiology of renovascular hypertension caused by FMD than for that caused by atherosclerosis.
Luminal narrowing leads to renal parenchymal damage and ischemic nephropathy in patients with FMD.69 This seems to be less important in patients with FMD than in those with atherosclerotic renal artery stenosis, however; the latter show more pronounced reductions of total kidney and cortical perfusion.70 In addition, renal perfusion correlates inversely with the degree of stenosis in FMD but not in atherosclerotic renal artery stenosis,70 further emphasizing that FMD hypertension is more truly renin dependent than hypertension due to atherosclerosis.71
The contralateral kidney may be damaged by exposure to hypertension in FMD.69 Deterioration of renal function in a patient with FMD affecting one renal artery suggests the development of bilateral stenosis, parenchymal disease, or both.
Natural History
Data with regard to stenosis progression and risk of deteriorating renal function in patients with FMD do exist,4,72–75 but they are scarcer than in patients with atherosclerotic renal artery stenosis. Progression is less severe in FMD than in atherosclerotic stenosis.3 About one fourth of subjects with asymptomatic FMD demonstrate hypertension within 4 years,4,5 and serial angiograms confirm FMD progression in up to 40% of cases.73,74 In one angiographic follow-up study of 42 patients, some progression occurred in all patients.75 Because angiography is not routinely performed in patients with FMD who have favorable clinical outcomes, these progression rates may be overestimated. FMD might also result in decreasing renal size75 and deterioration of renal function, although less often than in patients with atherosclerotic renal artery stenosis.22,71,76,77 Aneurysms8 and dissection8 are fairly frequent but complete vessel occlusion,78 renal infarction,75,79 and severe renal insufficiency,33,80 as well as regression of stenosis,81 have only been infrequently reported in patients with FMD.
Clinical Presentation
History and Physical Examination
Arterial hypertension of acute onset or high blood pressure that is increasingly difficult to treat suggests the presence of secondary hypertension—that is, a specific cause of blood pressure elevation, which can be identified in about 5% of adult hypertensive patients.19 Renovascular hypertension caused by one or more stenoses of the extrarenal arteries is the second most common cause of secondary hypertension (after renal parenchymal disease) and occurs in approximately 2% of adult patients with blood pressure elevation assessed in specialized centers.82 A physical sign suggesting renal artery stenosis is abdominal bruit with lateralization. In patients with either high-grade stenosis of a single kidney or bilateral disease, often with one renal artery occluded and the other stenosed, acute pulmonary edema may occur, with or without renal failure.83,84 Typically, these patients present with severe and rapid-onset “flash” pulmonary edema, which can also occur in FMD and may be confused with coronary syndromes.8,85 Among patients with renal artery stenosis, the absence of general atherosclerosis suggests that the stenosis is caused by FMD, whereas signs of atherosclerotic disease in other vessels indicate a greater possibility of an atherosclerotic cause.
Screening for Secondary Hypertension
The patient history can reveal acute-onset hypertension, concomitant flushing, or other paroxysmal symptoms. Physical examination may reveal abdominal bruits, and routine laboratory investigations may show signs of renal disease, hypokalemia, or hyperthyroidism. Secondary hypertension is also suggested by a severe blood pressure elevation, a sudden onset or worsening of hypertension, and blood pressure that responds poorly to appropriate doses of at least three drugs, including a diuretic.19,86 In these cases, specific diagnostic procedures for the evaluation of potential secondary hypertension should be considered, as outlined in Box 147-1.
Diagnostic Evaluation
In patients with suspected renovascular hypertension due to FMD, the same diagnostic tools as for arteriosclerotic renal artery stenosis are used. An overview and detailed description of these are given in Chapter 144. Here we comment only on special issues relating to FMD diagnosis.
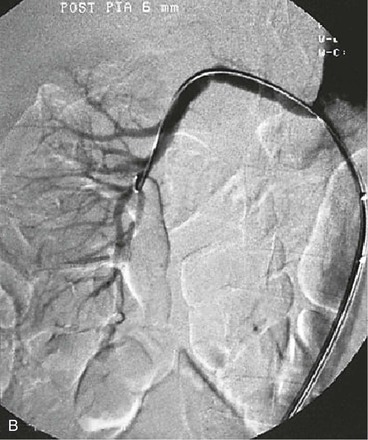
A limitation of renal artery duplex ultrasonography, MR angiography (MRA) and CT angiography (CTA) is their unreliability to exclude FMD because 20% to 25% of patients with FMD have branch lesions. Because MRA and CTA can identify vessels as small as 2 to 2.5 mm but have limited resolution for distal and intrarenal arteries,16,18,87 these techniques can be used to screen for possible FMD but not to exclude FMD. In terms of specificity and sensitivity, gadolinium-enhanced MRA and CTA are better than ultrasound and scintigraphy for the detection of renal artery stenosis,18,89,90 but intra-arterial digital subtraction angiography should be performed for confirmation or exclusion of FMD diagnosis. This invasive procedure is still the “gold standard” for the detection of renal artery FMD (Fig. 147-5),7,18 and is especially useful in the evaluation of branch vessel disease. Intra-arterial measurement of the pressure gradient over the stenosis, such as that performed before percutaneous transluminal angioplasty in the iliac arteries,91 is recommended before treatment of renal artery stenosis.88,89,92 Different methods have been used for such assessments,89,93,94 and there is no consensus regarding what level of mean or systolic pressure gradient indicates a hemodynamically significant renal artery stenosis. A renal-to-aortic pressure ratio less than 0.90 has been correlated with increased renin levels in the renal vein,95 suggesting a physiologically relevant stenosis. Pressure measurements have been used to determine the significance of renal artery stenosis due to FMD,33,88,96 and a mean pressure gradient over the stenosis of more than 10 mm Hg predicts a favorable response to dilatation. A persistent pressure gradient after an endovascular procedure indicates a higher risk of restenosis.
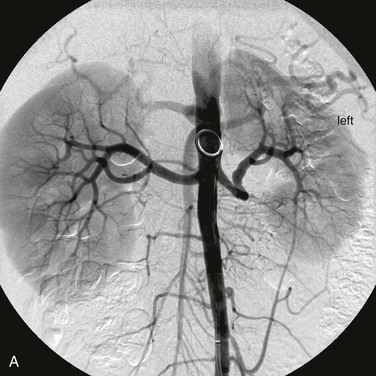
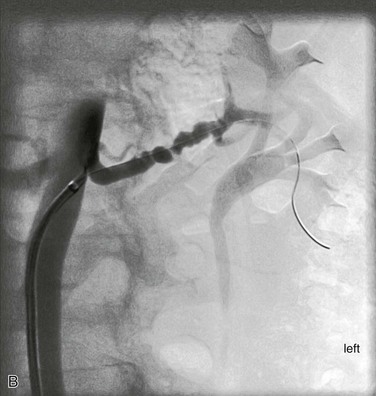
Figure 147-5 A, Aortogram in a hypertensive young man reveals only minor findings in the left renal artery. B, Selective angiogram from another angle shows the multifocal fibromuscular dysplastic lesion, over which a 60–mm Hg pressure gradient was noted. The patient’s hypertension was cured by percutaneous transluminal renal angioplasty.
Treatment Selection
The treatment options in renal artery FMD are medical, endovascular, and surgical. Treatment of patients with all forms of renovascular hypertension is controversial owing to the limited number of randomized, long-term-outcome trials comparing different therapeutic approaches as well as to the difficulty of predicting the blood pressure response to renal revascularization procedures in individual patients.65,82 In patients with FMD, invasive endovascular or surgical treatment should be considered in those whose hypertension cannot be controlled with antihypertensive drugs, in those who are intolerant of or noncompliant with medication, and in those with impaired renal function or ischemic nephropathy.7 To identify progressive disease in patients undergoing medical therapy only, blood pressure and renal function should be monitored regularly. Some writers also recommend that renal size be monitored by regular ultrasound examinations and that revascularization be recommended if it decreases by 1 cm or more.3
Medical Treatment
Prevention
In atherosclerotic renovascular disease, treatment with lifestyle modification, low-dose aspirin, and statins is warranted because of the high risk of progression of atherosclerotic lesions. In patients with FMD, who might have varying degrees of concomitant atherosclerosis or none at all, the indications for such treatment must be evaluated in the individual. The most important medical treatment in these patients is antihypertensive drugs.
Medication
Many controlled trials have convincingly shown that lowering blood pressure reduces cardiovascular morbidity and mortality.97 All patients with renovascular hypertension caused by FMD are therefore candidates for antihypertensive treatment in accordance with current European19,98 and American86 guidelines. Blood pressure should be reduced to 130 to 139 mm Hg systolic/90 mm Hg diastolic (130-139/80) in hypertensive patients, and further, to less than 130/80 mm Hg in patients with diabetes.
Current guidelines specify five different groups of first-line antihypertensive treatment: ACE inhibitors, angiotensin II receptor blockers (ARBs), beta blockers, calcium channel blockers, and diuretics.19,86 All of these drugs can be used in the treatment of renovascular hypertension due to FMD.
Thiazide diuretics and calcium antagonists can be used in appropriate doses, with the possible addition of a blocker of the renin-angiotensin system (ACE inhibitor or angiotensin II receptor blocker). ACE inhibitors and angiotensin II receptor blockers should not be taken during pregnancy, however. This treatment lowers blood pressure in the majority of patients with renovascular hypertension. In the patient with bilateral disease or stenosis of a single kidney, blockade of the renin-angiotensin system requires caution because these drugs’ dilatory effects on the efferent arterioles can compromise renal function, leading to a reduction in perfusion pressure beyond the stenosis. The reduction can lower the capillary pressure within the glomerulus to below the critical perfusion pressure.99 Slight increases in serum creatinine are common, but they normally revert when treatment is withdrawn. Acute renal failure occurring 1 to 14 days after the initiation of treatment has been described.100
Endovascular Treatment in Adults
At most institutions, open surgical procedures to optimize renal artery blood flow were the dominant invasive treatment for renovascular hypertension in patients with FMD until the beginning of the 1990s. Since then, percutaneous transluminal renal angioplasty (PTRA) has become the treatment of choice for renovascular hypertension due to FMD.101 Unlike in patients with atherosclerotic renovascular hypertension, progressive loss of renal function is uncommon in patients with FMD.102 Thus, the main reason for treating FMD is unsatisfactorily controlled hypertension, and treatment often leads to cure or improvement.
Technical Considerations
The technique for PTRA in FMD is basically the same as that used to treat atherosclerotic renal artery lesions (see Chapter 146). To reduce the number of “minor” complications after PTRA, many recommend a micropuncture technique of the common femoral artery, normally the right one. The puncture site position is controlled angiographically before catheters and guiding catheters are advanced, to ensure optimal compression of the puncture site after the procedure. Most centers are using guide-wire systems based on 0.014- or 0.018-inch guide wires to reduce vasospasm. All the components in such systems are thin and have low profiles. In patients with FMD, the right renal artery often takes off from the aorta proximally. Even in the right renal artery, which is often longer than normal in patients with FMD, the diversity of available guiding catheters usually allows catheterization from a femoral puncture. A brachial approach is seldom needed to get a better angle and/or “pushability” for renal catheterization.
Heparinization is the same as for other endovascular procedures. However, patients with FMD are often younger and have more pronounced vasoreactivity than those with atherosclerotic renal artery stenosis. Many patients with FMD are already receiving antihypertensive treatment with calcium channel blockers; if not, premedication with a short-acting dihydropyridine such as nifedipine can be used to reduce the risk of vasospasm. Even so, vasospasm in the kidney vasculature is so frequent in patients with FMD that one normally infuses 0.15 mg nitroglycerin into the renal artery just after catheterization in hemodynamically stable patients. If required, this dose can be repeated during the procedure.
After catetherization a diagnostic aortogram and selective renal angiograms at different angles are obtained (see Fig. 147-5). In addition, many centers routinely measure pressure gradients. Because there is no optimal tip-pressure catheter, pressure measurements are usually made through a 4F catheter. Balloons for 0.014- or 0.018-inch guide-wire systems are thin and normally pass easily into the renal artery.
In patients with FMD, the stenotic lesion is most often located in the middle part of the main renal artery. The diameter of the selected balloon is normally the same as the diameter of the unaffected proximal part of the renal artery. If the distal parts of the renal artery or its branches are affected, the use of multiple guide wires may be necessary. A kissing balloon technique can be used in branches.103 However, multiple guide wires or kissing balloons are normally not required, and inserting a balloon into the main artery first, followed by the branches, often achieves good results.104
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree