Chapter 150
Renovascular and Aortic Developmental Disorders
Jonathan L. Eliason, James C. Stanley
Developmental abdominal aortic disease is complex and often associated with stenotic lesions of the aortic branches to the kidneys and splanchnic organs. The etiology and clinical spectrum of this disease have been well defined in the past decade.1,2 A variety of interventions exist with the expectation that the majority of patients will benefit from appropriately planned and conducted vascular reconstructive procedures.
Developmental Abdominal Aortic Coarctation and Hypoplasia
Classification
Descriptions of abdominal aortic narrowings are varied in the existing literature. Abdominal aortic coarctation or segmental aortic hypoplasia accurately describes the majority of these lesions. The pattern of aortic narrowing distinct from isthmic coarctation involving the pediatric population was first categorized in 1963 by Sen et al,3 who used the phrase “middle aortic syndrome” to describe 16 patients, although the majority of patients included in this original report were actually older than 20 years of age. In this work, a distinction was made between coarctations arising above the diaphragm and those that were infradiaphragmatic. Many refer to this entity as the mid-aortic syndrome.
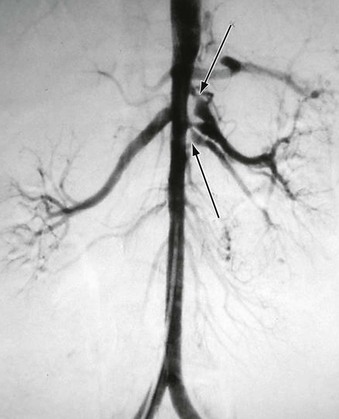
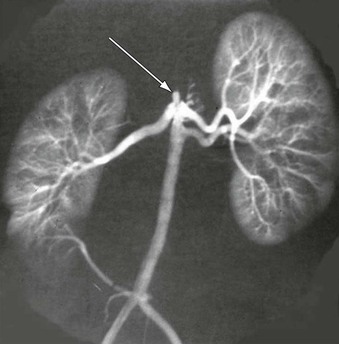
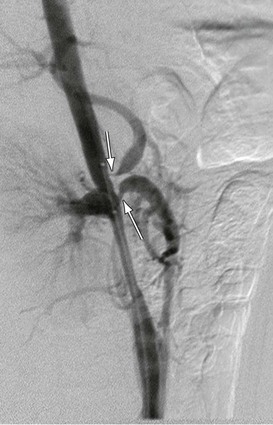
A more contemporary classification of developmental aortic narrowing is based on the most cephalad extent of the involved aorta, which distinguishes suprarenal (69%) from intrarenal (23%) and infrarenal (8%) narrowings (Figs. 150-1 to 150-3).1 Diffuse hypoplasia of the abdominal aorta is included in the spectrum of disease, but is often considered separately1,4 (Fig. 150-4).
Figure 150-1 A, Supararenal abdominal aortic coarctation (bracket) with superior mesenteric artery stenosis (arrow). B, Bilateral renal artery stenoses (arrows). Note common trunk of lower lumbar artery (arrow) on posterior projection. (From Stanley JC, et al: Abdominal aortic coarctation: surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg 48:1073-1082, 2008).
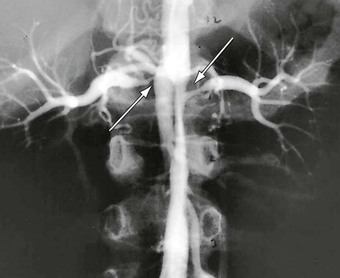
Figure 150-2 Intrarenal abdominal aortic coarctation with bilateral artery stenosis (arrows). (From Stanley JC, et al: Abdominal aortic coarctation: surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg 48:1073-1082, 2008.)
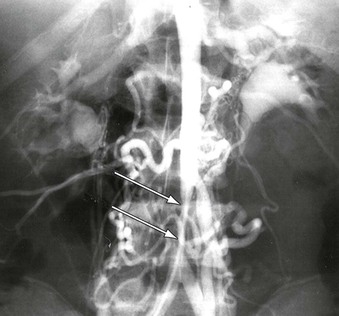
Figure 150-3 Infrarenal abdominal aortic coarctation manifest by tubular stenosis extending from a dilated inferior mesenteric artery to the aortic bifurcation. (From Stanley JC, et al: Pediatric arterial disease. in Coran AC, et al, editors: Pediatric surgery, ed 7, Philadelphia, 2012, Elsevier Saunders, pp. 1631-1645.)
Etiology and Pathogenesis
Embryonic
Overfusion of the two dorsal aortas during the fourth week of gestation is thought to be the cause of most developmental abdominal aortic narrowings. Before this event, the dorsal aortas extend along the entire length of the embryo, with each segment of the paired aortas containing dorsal intersegmental, lateral segmental, and ventral segmental branches. As the two dorsal aortas fuse, these branches persist, involute, dilate, or join together to form the named branches found at birth. A clinical finding supporting the overfusion theory of segmental aortic hypoplasia is the loss of paired lumbar arteries within a hypoplastic aortic segment, with only a single lumbar artery trunk exiting the dorsal aorta.5 A developmental etiology for aortic hypoplasia is also supported by the observation that multiple renal arteries to one or both kidneys occur in these patients with nearly twice the frequency that occurs in the general population.1,6,7 Aortic development occurs at the same time the multiple metanephric arteries involute to leave a single renal artery in the majority of fetuses. It is plausible that developmental aortic narrowings result in flow disturbances near what would usually be the principle renal artery; this diminishes its hemodynamic advantage and allows adjacent metanephric channels to persist as multiple or accessory renal arteries (Fig. 150-5).
Associated Genetic Syndromes and Infectious-Inflammatory Disorders
Certain genetic diseases or in utero events have been associated with abdominal aortic coarctations. The most frequent is neurofibromatosis-1 (NF-1).1,7–11 Others include William’s syndrome, Alagille syndrome, and tuberous sclerosis.12–16 It is theorized that each of these diseases results in interference of cell growth in vascular tissue or cellular death during development. The NF-1 phenotype deserves particular note. It is highly variable, and a wide spectrum of vascular involvement has also been noted. Although aneurysms, arteriovenous malformations, cardiac valvular anomalies, and direct neural tumor invasion or compression of vascular structures have been reported, hypertension due to renal artery stenotic disease or abdominal aortic narrowing is the most common presentation of NF-1, reflecting vascular involvement.17–20 It is difficult to find a unifying cause for this phenotype of NF-1, although the variable influence of modifying genes in addition to a primary NF-1 mutation, as well as alterations in the tissue specific elastin gene transcription may play a role.21,22 In our experience, more than 25% of 53 patients with abdominal aortic coarctation or segmental aortic hypoplasia carried a confirmed diagnosis of NF-1.1 This may not be reflective of the global prevalence of this disease. In another large series, of 31 patients who had NF-1–related vascular involvement, only four exhibited abdominal aortic coarctations.20
Maternal rubella during the first trimester is an infectious-inflammatory disorder that has been associated with abdominal aortic hypoplasia.23–25 Takayasu’s aortoarteritis is another recognized cause of abdominal aortic coarctation that must be differentiated from developmental narrowings, although this disease is more likely to involve the aortic arch or proximal descending thoracic aorta.26 Such an active or latent arteritis may be the most common cause of abdominal aortic narrowings encountered in the subcontinents.
Nondevelopmental abdominal aortic coarctation has been associated with umbilical artery catheterization during the neonatal period, although aortic thromboses and mycotic aneurysms have been reported more frequently than aortic coarctations.27,28 Nevertheless, knowledge of neonatal umbilical artery catheterization placement is an important part of the medical history to differentiate catheter-related abdominal aortic narrowings from developmental lesions.
Developmental Renal and Splanchnic Arterial Stenoses
Abdominal aortic narrowings are associated with stenoses of the splanchnic and renal arteries, in 87% and 62% of cases, respectively.1 Similar to abdominal aortic coarctation, pediatric renal artery stenoses represent a spectrum of diseases. Etiology of pediatric renal artery stenosis is most commonly developmental in North American Caucasian children,2,7 compared with more common inflammatory aortoarteritis and/or Takayasu-related stenoses that dominate reports from Asia, Africa, and South America.29–32 Larger clinical reports suggest that the causes of most developmental renal occlusive lesions are the same as those causing abdominal aortic coarctations,1,2 and that many of these patients have NF-1.2,20,33–35 The majority of renal artery narrowings are ostial in location, representing true hypoplastic stenoses. The affected arteries are very small, with derangements of all three vessel wall elements, including intimal proliferation, medial thinning, and excessive adventitial elastin (Fig. 150-6).
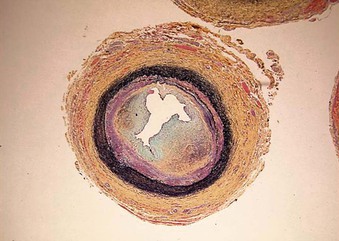
Figure 150-6 Typical developmental renal artery ostial stenosis exhibiting proliferation of intimal tissues, medial thinning, and excessive adventitial elastin (Movat stain).
Similar ostial stenoses of the celiac artery (CA) and superior mesenteric artery (SMA) occur in areas of abdominal aortic coarctations (Fig. 150-7). These stenotic narrowings usually are recognized most frequently during studies for suspected aortic or renal artery pathology. Developmental lesions appear related to embryonic events similar to those causing aortic and renal artery narrowings. These events likely affect the reorganization of the ventral segmental vessels associated with the cephalic roots of the vitelline arteries that form the CA and SMA. This results in an ostial stenosis or occlusion. Minimal CA and SMA narrowings at a young age may not increase in diameter as the child grows. In these circumstances, the artery’s origin becomes proportionately narrower as the adjacent artery becomes bigger, until the narrowing becomes a critical stenosis. This entire process suggests a major growth arrest of the aortic origins of these splanchnic arteries.
Clinical Manifestations
Occlusive disease affecting the renal arteries ranks as the third most prevalent cause of hypertension in children, behind parenchymal renal disease and coarctation of the thoracic aorta.36–38 The exact incidence of renovascular hypertension as a cause of secondary hypertension in the pediatric population is unknown, but is likely to account for 8% to 10% of those exhibiting marked blood pressure elevations.39 Although the overall incidence is low, renovascular disease accounts for a much larger proportion of hypertension in children than in the adult population.
Hypertension due to suprarenal or intrarenal aortic narrowings may be compounded by occlusive lesions of the renal artery. The resulting secondary hypertension in these cases is often refractory to simple pharmacologic control. Encounters with such patients who are receiving three or more antihypertensive agents is commonplace. Lower extremity fatigue with exercise due to abdominal aortic narrowing has been reported infrequently as the presenting complaint, and true claudication is rare.1
Despite the high rate of CA and SMA occlusive involvement with developmental narrowing of the abdominal aorta, in the experience of these authors, symptomatic intestinal ischemia affected only 6% of these cases.1 When these patients have functionally relevant splanchnic arterial narrowings, they present with postprandial intestinal angina manifest by periumbilical discomfort that lasts for the duration of small bowel transit of ingested food. These patients develop a food adversion and exhibit weight loss from a failure to eat.
Initial presentations of pediatric renovascular hypertension are quite varied and include asymptomatic high blood pressure, headache, seizure, epistaxis, visual disturbances, and acute renal insufficiency. The spectrum of neurologic dysfunction includes an isolated facial nerve paralysis, reported as Bell’s palsy.39,40 More chronic effects of elevated blood pressure may be evident by the arrest of kidney growth or atrophy with chronic renal insufficiency.41 When unrecognized and untreated, renovascular hypertension in children is associated with hemorrhagic stroke, hypertensive encephalopathy with impaired cognitive development, and failure to thrive. Poorly controlled hypertension also may result in severe diastolic dysfunction and left ventricular hypertrophy (LVH). When untreated, the resulting hypertensive cardiomyopathy can progress to heart failure. If the entire renal mass is involved, flash pulmonary edema associated with renal insufficiency may occur, although this is an uncommon complication.
Definition of Hypertension in Children
The National Health and Nutrition Examination Survey (NHANES) data are the basis for definitions of hypertension in children and adolescents. Blood pressure tables for the 50th, 90th, 95th, and 99th percentiles by sex, age, and height are included in The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents published by the U.S. Department of Health and Human Services (http://www.nhlbi.nih.gov/health/prof/heart/hbp/hbp_ped.pdf).42
Normal pressure is defined as systolic blood pressure (SBP) or diastolic blood pressure (DBP) that is less than the 90th percentile for sex, age, and height, whereas hypertension is defined as an average SBP or DBP that is greater than or equal to the 95th percentile for sex, age, and height on at least three separate occasions.
It is recommended that children older than 3 years of age who are seen in a medical setting have their blood pressure measured; the preferred method is by auscultation. It is critical that an appropriate sized cuff be used for the size of the child’s upper arm. Children younger than 3 years of age should also have blood pressure evaluations if congenital heart disease is identified, if there are known renal diseases or urologic malformations, if they require hospitalization, or if other systemic illnesses associated with hypertension are identified (e.g., neurofibromatosis, tuberous sclerosis, etc.).
The prevalence of LVH in children and adolescents with hypertension underscores the importance of recognizing and treating this disease. Although 43% of hypertensive children in one study were being treated with antihypertensive medication at the time of evaluation, LVH was present by echocardiogram in 41% using pediatric criteria and in 16% using adult criteria.41 Additional documented effects of pediatric hypertension include impaired cognition or failure to thrive.43–45
Diagnostic Evaluation
Imaging to accurately define renal artery stenotic disease requires significant detail.2,7,46,47 Because both computed tomography (CT) and magnetic resonance angiography (MRA) techniques have improved, some have relied heavily on these studies for the evaluation of patients. However, it is notable that even in the setting of what is considered a normal study by MRA or CT, if a strong suspicion of a renovascular lesion exists, then catheter-based digital subtraction angiography should be pursued.1 Many experienced clinicians still consider the latter to be the gold standard.2,47
Other screening studies such as nuclear renal scans that use angiotensin-converting enzyme (ACE) inhibitors and deep abdominal ultrasonography each have value, but remain too inconsistent to be used as a confirmatory diagnostic or prognostic test. In older patients, CTA can provide excellent image quality for follow-up, with the benefits of a noninvasive study being weighed against increased radiation exposure. Current noninvasive imaging techniques have improved considerably, with CTA and MRA providing good detail and seamless imaging of the chest, abdomen, and pelvis by utilizing 2- and 3-dimensional reformatted images. These studies can discriminate among aortic, renal, and combined causes of kidney malperfusion. Nevertheless, conventional angiography remains the most valuable diagnostic study for patients with this disease.
Medical Management
The degree of hypertension control with medical therapy is very important. Pharmacologic treatment of hypertension in a very young child may be difficult and requires frequent and careful monitoring. Failure of medical therapy to halt the progression or diminish LVH should be a reason to consider surgical or endovascular therapy.
All children with secondary hypertension should be treated. The goal of therapy is reduction of the pressure to the 95th percentile for sex, age, and height, unless LVH or other target organ damage is identified, in which case, the goal should be less than the 90th percentile. Although no specific recommendations regarding pharmacologic agents have been made, there are accepted classes of medications used in the management of hypertension in children ages 1 to 17 years old.
Antihypertensive Drug Treatment
Common classes of antihypertensive drugs for the ambulatory management of hypertensive children include: ACE inhibitors (benazepril, captopril, enalapril, fosinopril, lisinopril, quinapril); angiotensin-receptor-blockers (ARBs) (irbesartan, losartan); combined alpha and beta blockers (labetalol); beta blockers (atenolol, metoprolol, propranolol); combined beta blockers and diuretics (bisoprolol/hydrochlorothiazide [HCTZ]); central alpha agonists (clonidine); calcium channel blockers (amlodipine, felodipine, isradipine, extended-release nifedipine); diuretics (HCTZ, chlorthalidone, furosemide, spironolactone, triamterene, amiloride); peripheral alpha antagonists (doxazosin, prazosin, terazosin); and vasodilators (hydralazine, minoxidil).42 It is recommended that a single agent be initiated until target blood pressure is reached or until maximum dose, or a side effect of the drug are encountered, at which time an additional agent should be added.42
The nuances of medical pharmacologic treatment of renovascular hypertension are numerous and complicated, but two practical specifics deserve note. First, the ACE and/or ARB classes of medicine are potent antihypertensives, sometimes controlling blood pressures with monotherapy, whereas multiple agents from other classes of drugs fail. However, use of these agents may result in a decline in renal function with a rise in serum creatinine and blood urea nitrogen. This is most apt to occur when the blood flow to the entire renal mass is compromised (bilateral renal artery disease or unilateral disease in the presence of a solitary kidney). Discontinuation of these drugs usually results in return to baseline renal function. Second, the existence of re-bound hypertension and tachycardia seen with the sudden withdrawal of clonidine is particularly troublesome in children. When clonidine patches are used in a very young child, they may be accidentally detached, resulting in dramatic fluctuations in blood pressure and heart rate. A functioning, properly applied patch should be carefully monitored by those caring for these children. In these cases, one should confirm the adequacy of the patch placement before undergoing a complex search for the cause of an abrupt change in blood pressure control.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree