The relation between oxidative stress and coronary artery calcium (CAC) progression is currently not well described. The present study evaluated the relation among the biomarkers of oxidative stress, vascular dysfunction, and CAC. Sixty asymptomatic subjects participated in a randomized trial evaluating the effect of aged garlic extract plus supplement versus placebo and underwent measurement of CAC. The postcuff deflation temperature-rebound index of vascular function was assessed using a reactive hyperemia procedure. The content of oxidized phospholipids (OxPL) on apolipoprotein B-100 (apoB) particles detected by antibody E06 (OxPL/apoB), lipoprotein(a), IgG and IgM autoantibodies to malondialdehyde–low-density lipoprotein and apoB-immune complexes were measured at baseline and after 12 months of treatment. CAC progression was defined as an annual increase in CAC >15%. Vascular dysfunction was defined according to the tertiles of temperature-rebound at 1 year of follow-up. From baseline to 12 months, a strong inverse correlation was noted between an increase in CAC scores and increases in temperature-rebound (r 2 = −0.90), OxPL/apoB (r 2 = −0.85), and lipoprotein(a) (r 2 = −0.81) levels (p <0.0001 for all). The improvement in temperature-rebound correlated positively with the increases in OxPL/apoB (r 2 = 0.81, p = 0.0008) and lipoprotein(a) (r 2 = 0.79, p = 0.0001) but inversely with autoantibodies to malondialdehyde–low-density lipoprotein and apoB-immune complexes. The greatest CAC progression was noted with the lowest tertiles of increases in temperature-rebound, OxPL/apoB and lipoprotein(a) and the highest tertiles of increases in IgG and IgM malondialdehyde–low-density lipoprotein. In conclusion, the present results have documented a strong relation among markers of oxidative stress, vascular dysfunction, and progression of coronary atherosclerosis. Increases in OxPL/apoB and lipoprotein(a) correlated strongly with increases in vascular function and predicted a lack of progression of CAC.
Oxidized low-density lipoprotein (OxLDL) is primarily present in the vessel wall and has pro-atherogenic and pro-inflammatory properties. Previous in vitro and human studies have suggested that OxLDL might impair vascular function and play an important role in the pathogenesis of atherosclerosis. In addition, circulating OxLDL biomarkers, which can be measured directly as oxidative epitopes on apolipoprotein B-100 (apoB) or as autoantibodies or immune complexes to OxLDL, have been associated with various manifestations of cardiovascular disease. Oxidized phospholipids (OxPL) are major components of OxLDL and can be measured in plasma on apoB particles (OxPL/apoB) with antibody E06. Increased OxPL/apoB levels are associated with angiographically determined coronary artery disease, the presence and progression of carotid and femoral atherosclerosis, and the prediction of new cardiovascular events. The relation among OxLDL biomarkers, endothelial function, and progression of atherosclerosis has not been established, particularly in therapeutic studies. In the present study, we evaluated the interrelations of oxidative biomarkers, vascular function, and the progression of calcium in the context of a randomized clinical trial assessing the effect of aged garlic extract supplemented with B vitamins, folate, and l -arginine on coronary artery calcium (CAC).
Methods
The participants eligible for the present study were men and women, mean age 60 ± 9 years (range 40 to 79) with intermediate Framingham risk scores of 10% to 20% and CAC >30%. A total of 65 subjects receiving chronic statin therapy were randomized to commercially available aged garlic extract plus supplements consisting of aged garlic extract (500 mg), vitamin B 6 (25 mg), vitamin B 12 (200 μg), folate (600 μg), and l -arginine (200 mg) (Kyolic 108, Wakunaga Nutritional Supplement, Mission Viejo, California) versus equivalent placebos in a double-blind manner.
A total of 95 subjects were screened, 65 met the eligibility criteria and were recruited, and 60 completed the study and underwent baseline and 1-year follow-up CAC scanning and digital thermal mentoring of vascular function. Five participants did not complete the study protocol and were not included in the analysis. All participants were educated regarding a low-cholesterol diet and instructed to avoid any direct form of garlic and antioxidant supplementation. Subjects with established cardiovascular disease, stroke, diabetic retinopathy, peripheral vascular disease, underlying infection, cancer, systemic inflammation status, immunosuppression, end-stage renal or liver disease, creatinine >1.4 mg/dl, or triglycerides >400 mg/dl were excluded.
The body mass index, hip circumference, blood pressure, fasting blood glucose, high-sensitivity C-reactive protein, homocysteine, and lipid profile were obtained quarterly using standard techniques. A panel of OxLDL biomarkers was measured at baseline and at 12 months of follows-up as described in the following paragraphs. The study protocol and consent form were approved by the institutional review board committee of the Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center (Torrance, California).
The studies were performed with an E-Speed electron beam scanner (GE-Imatron, South San Francisco, California). The coronary arteries were imaged with 30 to 40 contiguous 3-mm slices (to ensure complete coronary coverage) during end-diastole using electrocardiographic triggering during a 30- to 35-second breath hold. CAC was considered present in a coronary artery when a density of >130 Hounsfield units was detected in ≥3 contiguous pixels (>1 mm 2 ) overlying that coronary artery and was quantified using the previously described Agatston scoring method. Patients were classified as having CAC progression if the CAC increased >15% during the 1-year study period and as not having CAC progression, if the progression was <15%.
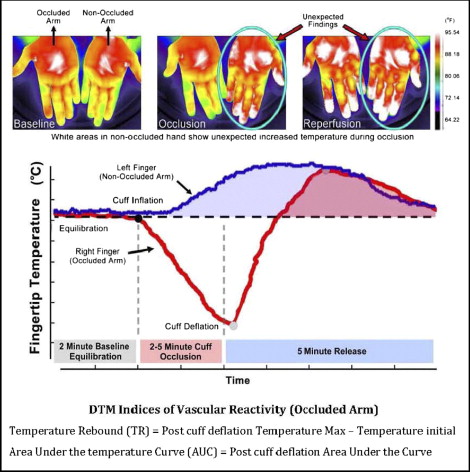
All digital thermal mentoring measurements were performed in the morning in a quiet, dimmed room at a controlled ambient temperature of 23.5° to 25.0°C. The studies were conducted after an overnight fast of ≥10 hours (water was permitted) and abstinence from tobacco, alcohol, caffeine, vasoactive medications, exercise, high-fat foods, and vitamin C. The measurements were obtained with the subjects in the supine position and after 30 minutes of rest. Each patient’s blood pressure in the control arm was recorded with the patient in the sitting position 5 minutes before the digital thermal mentoring test (IntelliSense Professional Digital Blood Pressure Monitor, HEM-907XL, Omron, Schaumberg, Illinois). Digital thermal mentoring of both hands was obtained during 5 minutes of stabilization, 5 minutes of cuff inflation to 50 mm Hg greater than systolic blood pressure, and 5 minutes of deflation using an automated, operator-independent protocol (VENDYS, Endothelix, Houston, Texas). Thermal changes during the 5-minute arm cuff-induced reactive hyperemia test were monitored continuously in the fingertip of both the occluded and nonoccluded arms using VENDYS software. The device consists of a computer-based thermometry system (0.006°C thermal resolution) with 2-fingertip resistance temperature detector fast response probes designed to minimize the skin-probe contact area and fingertip pressure that attach to the pulp of the index finger on both hands. The system includes a common automated sphygmomanometer cuff, cuff-inflation pump, and release valve to permit noninvasive measurement of the arterial pressure and the control of occlusive hyperemia. Dual-channel temperature data are simultaneously acquired at a 1-Hz sampling rate. The equations for postcuff deflation temperature rebound and area under the temperature curve are shown in Figure 1 . Vascular dysfunction was defined according to the tertiles of temperature rebound at 1 year of follow-up: persistent (temperature rebound ≤0.7), borderline (temperature rebound >0.7 but <1.1), or improved (temperature rebound ≥1.1).
The OxPL/apoB levels were measured as described previously in detail using chemiluminescent enzyme-linked immunosorbent assay and the murine monoclonal antibody E06, which binds to the phosphorylcholine head group of oxidized, but not native, phospholipids. The data are presented as the relative light units per 100 ms. The OxPL/apoB measurement is designed to be independent of apoB (and LDL cholesterol) levels. Chemiluminescence enzyme-linked immunosorbent assays were also used to measure IgG and IgM autoantibodies to malondialdehyde (MDA)-LDL and apoB-immune complexes, as described previously.
Plasma lipoprotein(a) levels was measured using a chemiluminescent enzyme-linked immunosorbent assay with monoclonal antibody LPA4, as described previously.
All statistical analyses were performed using Statistical Package for Social Sciences, version 16.0 (SPSS, Chicago, Illinois). All continuous data are presented as the mean ± SD, and all categorical data are reported as percentages or absolute numbers. Student’s t tests and chi-square tests were used to assess the differences between groups. The percentage of change in the CAC was calculated as follows: (CAC score at 1 year−CAC score at baseline/CAC score at baseline) × 100. The nonlinear correlation between progression, temperature-rebound, OxPL/apoB ratio, and lipoprotein(a) were measured by linear regression analysis with an exponential fit. The association between CAC progression and risk factors was analyzed using logistic regression analyses of the entire cohort, irrespective of the treatment assignment. These analyses were adjusted for demographics, age, gender, conventional cardiovascular risk factors, and statin and aged garlic extract plus supplements therapy. The results are reported as the odds ratio for the logistic regression analysis.
Results
The patient cohort was defined as those with and without CAC progression according to an annual change of >15% or <15%. The baseline clinical characteristics, lipid variables, oxidative biomarkers, digital thermal mentoring of vascular function, and CAC were similar between those with and without CAC progression ( Table 1 ).
| Variable | CAC | p Value | |
|---|---|---|---|
| No Progression (n = 29) | Progression (n = 31) | ||
| Age (years) | 61.2 ± 9.2 | 61.1 ± 9.7 | 0.96 |
| Men | 23 (79%) | 24 (77%) | 0.81 |
| Diabetes mellitus ⁎ | 1 (4%) | 4 (12%) | 0.38 |
| Hypertension † | 11 (37%) | 19 (62%) | 0.04 |
| Hypercholesterolemia ‡ | 22 (75%) | 26 (84%) | 0.55 |
| Statin therapy | 29 (100%) | 31 (100%) | 1.00 |
| Antihypertensive therapy | 10 (89%) | 27 (87%) | 0.92 |
| Family history of CHD § | 20 (67%) | 20 (65%) | 0.75 |
| Smoker | 11 (37%) | 11 (35%) | 0.75 |
| Body mass index (kg/m 2 ) | 27.6 ± 4.8 | 27.9 ± 5.1 | 0.82 |
| CAC score | 296 ± 79% | 345 ± 61% | 0.56 |
| Low-density lipoprotein (mg/dl) | 117 ± 27 | 114 ± 34 | 0.71 |
| High-density lipoprotein (mg/dl) | 48 ± 10 | 44 ± 8 | 0.14 |
| Triglycerides (mg/dl) | 125 ± 32 | 130 ± 35 | 0.39 |
| Total cholesterol (mg/dl) | 188 ± 35 | 184 ± 37 | 0.68 |
| C-reactive protein (mg/L) | 3.1 ± 4.1 | 2.9 ± 3.2 | 0.81 |
| Homocysteine (mg/dl) | 10.7 ± 2.8 | 10.4 ± 2.7 | 0.63 |
| Temperature rebound (°C) | 0.67 ± 0.18 | 0.66 ± 0.21 | 0.95 |
| Area under temperature curve | 105 ± 13 | 112 ± 9 | 0.69 |
| OxPL/apoB (RLU/ms) | 3,789 ± 763 | 3,428 ± 863 | 0.75 |
| IgG MDA-LDL (RLU/ms) | 3,848 ± 281 | 3,976 ± 364 | 0.82 |
| IgM MDA-LDL (RLU/ms) | 1,916 ± 216 | 1,874 ± 215 | 0.92 |
| IgG apoB-immune complexes (RLU/ms) | 4,783 ± 501 | 4,704 ± 274 | 0.95 |
| IgM apoB-immune complexes (RLU/ms) | 2,254 ± 260 | 2,434 ± 319 | 0.71 |
| Lipoprotein(a) (mg/dl) | 17.1 ± 6.1 | 16.1 ± 5.8 | 0.68 |
⁎ Self-reported diagnosis of diabetes (type 1 or 2) or prescribed medication for diabetes.
† Self-reported diagnosis of hypertension, prescribed medication for hypertension, or current blood pressure >140 mm Hg systolic or >90 mm Hg diastolic (>130/80 mm Hg if diabetic).
‡ Self-reported diagnosis of high cholesterol, prescribed medication for high cholesterol, or current total cholesterol >200 mg/dl.
§ First degree relative; women <65 years old, men <55 years old.
The absolute changes in CAC, temperature rebound, laboratory variables, and OxLDL biomarkers are listed in Table 2 . The absolute change in the CAC score was greater in those with CAC progression than in those without CAC progression (74.1 ± 15.2% vs 6.3 ± 4.4%, p = 0.0001). The patients with CAC progression had a lower temperature rebound and area under the temperature curve. Those with CAC progression also had significantly greater increases in LDL cholesterol and homocysteine and decreases in high-density lipoprotein cholesterol than those without CAC progression. Compared to the baseline values, the absolute levels of OxPL/apoB and lipoprotein(a) increased significantly more in those without CAC progression than in those with CAC progression at 12 months. In contrast, IgG and IgM MDA-LDL and apoB-immune complexes levels decreased more in those without CAC progression than in those with CAC progression ( Table 2 ).
| Variable | CAC | p Value | |
|---|---|---|---|
| No Progression (n = 29) | Progression (n = 31) | ||
| CAC score | 6.3 ± 4.4% | 74.1 ± 15.2% | 0.0001 |
| Temperature rebound (°C) | 0.28 ± 0.08 | 0.02 ± 0.09 | 0.042 |
| Area under temperature curve | 50.5 ± 14.7 | 8.4 ± 13.3 | 0.038 |
| C-reactive protein (mg/L) | −1.5 ± 1.9 | −1.1 ± 1.6 | 0.66 |
| Homocysteine (mg/dl) | −0.69 ± 2.24 | 0.42 ± 2.1 | 0.047 |
| Low-density lipoprotein (mg/dl) | −8.9 ± 4.2 | 5.2 ± 5.1 | 0.034 |
| High-density lipoprotein (mg/dl) | 7.05 ± 1.36 | 3.19 ± 1.03 | 0.042 |
| Triglycerides (mg/dl) | −6.7 ± 6.9 | 3.45 ± 9.2 | 0.34 |
| Total cholesterol (mg/dl) | −2.9 ± 4.1 | 9.8 ± 6.1 | 0.07 |
| OxPL/apoB (RLU/ms) | 4,007 ± 1692 | 2,820 ± 1447 | 0.0001 |
| Lipoprotein (a) (mg/dl) | 18.2 ± 3.2 | 8.9 ± 2.9 | 0.005 |
| IgG MDA-LDL (RLU/ms) | −644 ± 396 | 1,259 ± 436 | 0.003 |
| IgM MDA-LDL (RLU/ms) | −990 ± 392 | 269 ± 273 | 0.001 |
| IgG apoB-immune complexes (RLU/ms) | −820 ± 208 | 634 ± 213 | 0.003 |
| IgM apoB-immune complexes (RLU/ms) | −1,258 ± 363 | 545 ± 279 | 0.002 |
After adjustment for age, gender, conventional cardiovascular risk factors, statin therapy, and active aged garlic extract plus supplements therapy by logistic regression analysis, the odds ratio of the greatest versus 2 lower tertiles of temperature rebound, OxPL/apoB, and lipoprotein(a) was 0.33, 0.48, and 0.52 for those with CAC progression compared to those without CAC progression, respectively. In addition, the odds ratio of the greatest versus 2 lower tertiles of IgG and IgM autoantibodies to MDA-LDL and apoB-immune complexes was 2.04, 2.49, 2.23, and 2.79 for those with CAC progression, respectively. Multivariate logistic regression analysis showed that the digital thermal mentoring indexes and OxLDL biomarkers were each independent predictors of CAC progression. High-sensitivity C-reactive protein was not an independent predictor of CAC progression ( Table 3 ).
| Model | CAC Progression ⁎ | |
|---|---|---|
| Odds Ratio (95% CI) | p Value | |
| Odds of temperature rebound | 0.33 (0.170.81) | 0.01 |
| Odds of area under temperature curve | 0.34 (0.19–0.84) | 0.01 |
| Odds of LDL cholesterol | 1.23 (1.05–2.34) | 0.03 |
| Odds of HDL cholesterol | 0.72 (0.43–0.96) | 0.03 |
| Odds of triglycerides | 1.14 (0.81–1.12) | 0.30 |
| Odds of total cholesterol | 1.27 (0.80–1.64) | 0.10 |
| Odds of homocysteine | 1.34 (1.03–3.21) | 0.04 |
| Odds of C-reactive protein | 1.08 (0.79–1.34) | 0.30 |
| Odds of OxPL/apoB | 0.48 (0.27–0.91) | 0.01 |
| Odds of IgG MDA-LDL | 2.04 (1.12–3.28) | 0.01 |
| Odds of IgM MDA-LDL | 2.49 (1.09–3.39) | 0.01 |
| Odds of IgG apoB-immune complexes | 2.23 (1.11–4.35) | 0.02 |
| Odds of IgM apoB-immune complexes | 2.79 (1.16–4.27) | 0.01 |
| Odds of lipoprotein(a) | 0.52 (0.29–0.93) | 0.02 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


