In the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), random allocation of rosuvastatin compared to placebo among primary prevention patients with a low-density lipoprotein cholesterol level of <130 mg/dl and a high-sensitivity C-reactive protein (hs-CRP) level of ≥2 mg/L resulted in a highly significant 44% reduction in major vascular events. However, the relation of baseline hs-CRP levels to risk within JUPITER has not previously been described and has been an area of controversy for study interpretation. As reported in the present study for the first time, despite enrolling patients with a constrained range of values, increasing baseline hs-CRP levels within JUPITER were nonetheless associated with increasing vascular risk in analyses treating hs-CRP as a continuous variable, as an ordinal variable, and as a threshold variable. As anticipated, the relative risk reduction associated with rosuvastatin was similar in magnitude across the tertile and threshold levels of entry hs-CRP. In conclusion, as the absolute risk increased with increasing hs-CRP, the absolute risk reduction associated with rosuvastatin within JUPITER was also greatest among those with the greatest entry hs-CRP levels.
For the 17,802 primary prevention patients with low-density lipoprotein (LDL) cholesterol levels <130 mg/dl and high-sensitivity C-reactive protein (hs-CRP) levels of ≥2 mg/L in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, random allocation to rosuvastatin was associated with a 54%, 48%, 46%, 43%, and 20% reduction in myocardial infarction, stroke, the need for angioplasty or bypass surgery, venous thrombosis, and all-cause mortality, respectively. These effects were consistent in all subgroups evaluated, including among women and men, minority populations, at all levels of Framingham Risk, and among those with and without the metabolic syndrome. However, the relation of baseline hs-CRP levels to risk within JUPITER has not been previously described and has been an area of controversy for study interpretation. Because the trial design had a highly constrained range of hs-CRP values at study entry, one might have anticipated minimal, if any, relation between baseline hs-CRP and the subsequent risk of vascular events. In contrast, a recent meta-analysis of 54 prospective cohort studies has shown that a threefold increase in hs-CRP levels is associated with a highly significant 1.6-fold increase in vascular risk after adjustment for age and gender. This suggests that even within the range evaluated in JUPITER, increased levels might be associated with increased risk. These issues are clinically relevant, because new guidelines for primary prevention have already been published that advocate the use of hs-CRP to better target statin therapy and because more physicians are considering statins for primary prevention when hs-CRP levels are increased, even when LDL cholesterol levels are low.
Methods
The study population was derived from JUPITER. JUPITER was a randomized, double-blind, placebo-controlled trial designed to investigate whether rosuvastatin 20 mg/day compared to placebo decreases the rate of first-ever cardiovascular events among apparently healthy men >50 years old and women >60 years old with LDL cholesterol <130 mg/dl at increased vascular risk because of a hs-CRP level of ≥2 mg/L. The full details of the trial protocol, procedures, and methods of confirming the clinical end points and ascertaining adverse events have been previously presented. The trial exclusion criteria included treatment with any lipid-lowering therapy in the 6 weeks preceding randomization, current use of postmenopausal hormonal replacement therapy, evidence of hepatic dysfunction, creatinine >2.0 mg/dl, diabetes, uncontrolled hypertension, or hypothyroidism, a history of malignancy within 5 years, or another serious medical condition that might compromise patient safety or successful completion of the study. Because a core scientific hypothesis of JUPITER was related to underlying low-grade inflammation as evidenced by hs-CRP levels of ≥2.0 mg/L, subjects with inflammatory conditions such as severe arthritis, lupus, or inflammatory bowel disease were excluded. The ClinicalTrial.gov identifier was NCT00239681 .
For the purposes of the present analysis, the relation of the entry hs-CRP to subsequent vascular risk was evaluated using 3 strategies. First, log-transformed levels of the entry hs-CRP were treated as a continuous variable, and Cox proportional hazard models were used to evaluate an association with subsequent risk. Second, to evaluate for evidence of ordinal effects, the participants were classified according to increasing tertiles of hs-CRP at study entry. Finally, to assess for any evidence of a threshold effect, the analyses were repeated for those with baseline hs-CRP levels of >2, >4, >6, >8, and >10 mg/L at study entry. All analyses were performed on a gender-specific basis and all evaluated for relations in the placebo group, rosuvastatin group, and the combined total JUPITER population. In the tertile and threshold analyses, both relative and absolute risk reductions were also calculated in comparisons within each stratum for those allocated to rosuvastatin compared to placebo. Evaluations were performed separately for (1) the JUPITER primary end point of major vascular events (nonfatal myocardial infarction, nonfatal stroke, arterial revascularization, hospitalization for unstable angina, or cardiovascular mortality); (2) the end point of major vascular events and all-cause mortality; and (3) the end point of major vascular events, venous thromboembolism, and all-cause mortality. The latter end point was included because a prespecified analysis of the JUPITER trial included an evaluation of venous thromboembolism. All p values are 2-tailed, and all confidence intervals (CIs) were calculated at the 95% level.
JUPITER was an investigator-initiated trial. The sponsor of the study collected the trial data and monitored the study sites but had no access to unblinded data until after the drafting of the trial primary report. All statistical analyses were done by the investigators and the academic study statistician (RJG). Both the trial principle investigator (PMR) and the academic study statistician (RJG) had full access to all study data and had final responsibility for the decision to submit these data for publication.
Results
In the continuous analysis, the entry levels of log-transformed hs-CRP were significantly associated with subsequent vascular events among men who constituted approximately 2/3 of the study cohort. For the trial primary end point, each 1-U increase in log hs-CRP was associated with a 1.3-fold increase in vascular risk among these men (95% CI 1.1 to 1.5, p = 0.002), an effect that was modestly attenuated after adjustment for age, baseline LDL and high-density lipoprotein cholesterol levels, body mass index, blood pressure, smoking status, family history of premature atherosclerosis, and randomized treatment assignment (adjusted hazard ratio 1.2 per log hs-CRP, 95% CI 1.0 to 1.4, p = 0.02). For the combined end point of vascular events plus all-cause mortality, the adjusted hazard ratio per log hs-CRP was 1.4 (95% CI 1.2 to 1.5, p <0.0001). For the end point of vascular events, venous thrombosis, plus all-cause mortality, the adjusted hazard ratio per log hs-CRP was also 1.4 (95% CI 1.2 to 1.5, p <0.0001). For women, who comprised a smaller sample size, similar effects were noted in the fully adjusted analyses for the combined end point of vascular events plus all-cause mortality (hazard ratio 1.4, 95% CI 1.2 to 1.6, p = 0.0003) and for the combined end point of vascular events, venous thrombosis, plus all-cause mortality (hazard ratio 1.3, 95% CI 1.1 to 1.6, p = 0.0003). In the fully adjusted analyses for vascular events alone, in which the power was limited among women, a nonsignificant trend was observed (hazard ratio 1.1, 95% CI 0.85 to 1.4, p = 0.5).
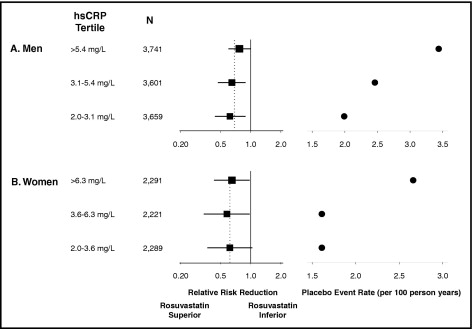
In the tertile analysis, the rates among men for the primary end point increased from 0.99 per 100 person-years in the lowest tertile to 1.42 per 100 person-years in the greatest tertile (p trend across tertiles = 0.023; Table 1 ). For the end point of vascular events plus all-cause mortality, the rate across the 3 tertiles of baseline hs-CRP was 1.61, 2.03, and 3.05 per 100 person-years, respectively (p trend <0.0001). For the end point that also included venous thromboembolism, the corresponding rates were 1.84, 2.18, and 3.29 (p trend <0.0001). Similar effects were also present within the rosuvastatin group and placebo group. As also listed in Table 1 , the relative risk reductions associated with rosuvastatin were statistically significant at all tertiles of hs-CRP, with no modification of magnitude of effect. Thus, the greatest absolute risk reduction among men enrolled in the JUPITER was observed among those with the greatest tertile of baseline hs-CRP.
| Tertile | hs-CRP (mg/L) | Patients (n) | Incidence Rate per 100 Person-Years | RR | 95% CI | p Value | ||
|---|---|---|---|---|---|---|---|---|
| Total Cohort | Rosuvastatin | Placebo | ||||||
| Primary end point | ||||||||
| Highest | >5.4 | 3,741 | 1.42 | 1.09 | 1.73 | 0.63 | 0.43–0.93 | 0.02 |
| Middle | 3.1–5.4 | 3,601 | 1.24 | 0.98 | 1.49 | 0.66 | 0.44–1.00 | 0.048 |
| Lowest | 2.0–3.1 | 3,659 | 0.99 | 0.59 | 1.39 | 0.43 | 0.26–0.69 | 0.0003 |
| p for trend | 0.023 | 0.042 | 0.20 | |||||
| Primary end point plus all-cause mortality | ||||||||
| Highest | >5.4 | 3,741 | 3.05 | 2.65 | 3.44 | 0.77 | 0.60–1.00 | 0.045 |
| Middle | 3.1–5.4 | 3,601 | 2.03 | 1.59 | 2.46 | 0.65 | 0.47–0.89 | 0.007 |
| Lowest | 2.0–3.1 | 3,659 | 1.61 | 1.24 | 1.99 | 0.62 | 0.44–0.89 | 0.008 |
| p for trend | <0.0001 | <0.0001 | <0.0001 | |||||
| Primary end point plus venous thrombosis plus all-cause mortality | ||||||||
| Highest | >5.4 | 3,741 | 3.29 | 2.84 | 3.72 | 0.76 | 0.59–0.98 | 0.03 |
| Middle | 3.1–5.4 | 3,601 | 2.18 | 1.65 | 2.70 | 0.61 | 0.45–0.84 | 0.002 |
| Lowest | 2.0–3.1 | 3,659 | 1.84 | 1.44 | 2.25 | 0.64 | 0.46–0.89 | 0.007 |
| p for trend | <0.0001 | <0.0001 | <0.0001 | |||||
Table 2 lists similar analyses across hs-CRP tertiles at study entry for women. In this subgroup, for the primary JUPITER end point in which the total number of events was modest for women, the relation was less clear. However, for the 2 combined end points, for which more power was available to detect true effects, similar relations between the entry hs-CRP and subsequent vascular risk was seen among women. For example, for the JUPITER end point of vascular events, venous thrombosis, and all-cause mortality, the rate from lowest to greatest tertile of hs-CRP among women was 1.39, 1.42, and 2.36 per 100 person-years of exposure (p trend = 0.0002). Just as with the men, rosuvastatin resulted in similar relative risk reductions in all hs-CRP strata among women such that the greatest absolute risk reductions were again seen among those with the greatest baseline hs-CRP levels. The results of the tertile analyses for men and women are summarized graphically in Figure 1 .
| Tertile | hs-CRP (mg/L) | Patients (n) | Incidence Rate per 100 Person-Years | RR | 95% CI | p Value | ||
|---|---|---|---|---|---|---|---|---|
| Total Cohort | Rosuvastatin | Placebo | ||||||
| Primary end point | ||||||||
| Highest | >6.3 | 2,291 | 0.93 | 0.71 | 1.16 | 0.62 | 0.33–1.15 | 0.12 |
| Middle | 3.6–6.3 | 2,221 | 0.65 | 0.51 | 0.81 | 0.63 | 0.30–1.31 | 0.21 |
| Lowest | 2.0–3.6 | 2,289 | 0.81 | 0.48 | 1.16 | 0.41 | 0.20–0.82 | 0.009 |
| p for trend | 0.34 | 0.26 | 0.74 | |||||
| Primary end point plus all-cause mortality | ||||||||
| Highest | >6.3 | 2,291 | 2.15 | 1.69 | 2.62 | 0.65 | 0.43–0.98 | 0.036 |
| Middle | 3.6–6.3 | 2,221 | 1.27 | 0.94 | 1.61 | 0.58 | 0.34–0.98 | 0.04 |
| Lowest | 2.0–3.6 | 2,289 | 1.30 | 1.00 | 1.61 | 0.62 | 0.37–1.04 | 0.07 |
| p for trend | 0.0004 | 0.017 | 0.009 | |||||
| Primary end point plus venous thrombosis plus all-cause mortality | ||||||||
| Highest | >6.3 | 2,291 | 2.36 | 1.87 | 2.85 | 0.66 | 0.45–0.98 | 0.04 |
| Middle | 3.6–6.3 | 2,221 | 1.42 | 1.07 | 1.79 | 0.59 | 0.36–0.98 | 0.04 |
| Lowest | 2.0–3.6 | 2,289 | 1.39 | 1.04 | 1.75 | 0.59 | 0.36–0.99 | 0.04 |
| p for trend | 0.0002 | 0.008 | 0.007 | |||||
As listed in Table 3 , for men, the rates for the JUPITER primary end point and the 2 prespecified combined end points also increased as the thresholds for entry hs-CRP increased up to >10 mg/L. For example, the rate per 100 person-years among men for the primary end point was 1.21, 1.38, 1.42, 1.47, and 1.57 for those with an entry hs-CRP level of ≥2, ≥4, ≥6, ≥8, and ≥10 mg/L, respectively. As also listed in Table 3 , similar effects were observed in the analyses limited to those taking rosuvastatin or taking placebo. The relative risk reductions associated with rosuvastatin were generally consistent across all subgroups, such that the greatest absolute risk reduction was observed among those with the greatest entry hs-CRP level. As listed in Table 4 , similar threshold effects were seen for women. The results of the threshold analyses for men and women are summarized graphically in Figure 2 .
| hs-CRP (mg/L) | Patients (n) | Incidence Rate per 100 Person-Years | RR | 95% CI | p Value | ||
|---|---|---|---|---|---|---|---|
| Total Cohort | Rosuvastatin | Placebo | |||||
| Primary end point | |||||||
| ≥2 | 11,001 | 1.21 | 0.88 | 1.54 | 0.58 | 0.45–0.73 | <0.0001 |
| ≥4 | 5,593 | 1.38 | 1.16 | 1.59 | 0.73 | 0.53–1.00 | 0.047 |
| ≥6 | 3,266 | 1.42 | 1.10 | 1.72 | 0.64 | 0.42–0.96 | 0.03 |
| ≥8 | 2,138 | 1.47 | 0.90 | 2.03 | 0.44 | 0.26–0.75 | 0.002 |
| ≥10 | 1,563 | 1.57 | 1.07 | 2.06 | 0.52 | 0.29–0.93 | 0.02 |
| Primary end point plus all-cause mortality | |||||||
| ≥2 | 11,001 | 2.23 | 1.82 | 2.64 | 0.69 | 0.58–0.82 | <0.0001 |
| ≥4 | 5,593 | 2.76 | 2.44 | 3.06 | 0.80 | 0.64–0.99 | 0.044 |
| ≥6 | 3,266 | 3.10 | 2.74 | 3.45 | 0.79 | 0.60–1.04 | 0.09 |
| ≥8 | 2,138 | 3.39 | 2.62 | 4.15 | 0.63 | 0.45–0.88 | 0.005 |
| ≥10 | 1,563 | 3.73 | 2.83 | 4.60 | 0.61 | 0.43–0.89 | 0.008 |
| Primary end point plus venous thrombosis plus all-cause mortality | |||||||
| ≥2 | 11,001 | 2.44 | 1.97 | 2.90 | 0.68 | 0.57–0.80 | <0.0001 |
| ≥4 | 5,593 | 2.96 | 2.57 | 3.34 | 0.77 | 0.62–0.95 | 0.015 |
| ≥6 | 3,266 | 3.37 | 2.95 | 3.78 | 0.78 | 0.60–1.01 | 0.06 |
| ≥8 | 2,138 | 3.72 | 2.85 | 4.58 | 0.62 | 0.45–0.85 | 0.003 |
| ≥10 | 1,563 | 4.09 | 3.02 | 5.12 | 0.59 | 0.42–0.84 | 0.003 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


