Adjunctive therapy with abciximab during primary percutaneous coronary intervention (PPCI) in patients with ST-elevation myocardial infarction (STEMI) determines a better short-term outcome compared to placebo. Tirofiban and eptifibatide represent a valid option with lower cost, but these have been less studied. The aim of the present study was to combine all randomized trials and registries to demonstrate the noninferiority of tirofiban and eptifibatide compared to abciximab in patients with STEMI treated with PPCI. We identified 6 randomized trials and 4 registries. Overall, 4,653 received small molecules and 2,696 abciximab, and the rate of combined death and nonfatal reinfarction did not differ (4.6% vs 4.5%, odds ratio 0.99, 95% confidence interval [CI] 0.78 to 1.27, p = 0.95) up to 30 days of follow-up, with an absolute difference of 0.1% (95% CI −1.06 to 0.8). Because the noninferiority limit was set at +1.5%, and because the upper point estimate (0.8%) of the 95% CI did not cross the prespecified limit, the noninferiority of the small molecules was documented. In-hospital major bleeding was also similar (8.8% vs 6.1%, odds ratio 0.92, 95% CI 0.75 to 1.13, p = 0.43). Sensitivity analysis comparing randomized trials to registries and tirofiban or eptifibatide to abciximab did not show any significant differences. In conclusion, our results documented noninferiority of “small molecules” compared to abciximab and, therefore, support their alternative use as adjunctive therapy during PPCI for patients with STEMI.
Primary percutaneous coronary intervention (PPCI) is the preferred method for reperfusion of the myocardium in patients with ST-elevation myocardial infarction (STEMI). Because platelets play a pivotal role in the pathogenesis of STEMI, therapies targeted to inhibit platelet activation are crucial to successful PPCI. Adjunctive treatment with the glycoprotein IIb/IIIa receptor antagonist abciximab (ABX) has been studied in small to medium trials and meta-analysis, showing a significant decrease of major cardiovascular events, without a significant increase in major bleeding. However, the relevant cost burden of ABX represents a major issue against its routine implementation. “Small molecule” (SM) glycoprotein IIb/IIIa receptor antagonists, e.g., tirofiban and eptifibatide, might represent a valid option with significant cost decrease, but these have been less extensively studied in the STEMI population. Despite lack of evidence, patients with STEMI are being treated with SM in many countries. To substantiate such “real-word” practice we sought to determine whether SM would be at least as effective as ABX in decreasing ischemic events by aggregating all trial and registry data.
Methods
We identified all randomized clinical trials (RCTs) and registries, published and unpublished, comparing SM to ABX treatment during PPCI. The literature was scanned by formal searches of electronic databases (MEDLINE, PubMed) from January 1995 to December 2008 and of scientific session abstracts in Circulation , Journal of the American College of Cardiology , and European Heart Journal . Conference proceedings for the scientific sessions of the American College of Cardiology, the American Heart Association, Transcatheter Cardiovascular Therapeutics, and the European Society of Cardiology and Web sites (including www.cardiosource.com , www.TCTMD.com , and www.the-heart.org ) were also evaluated. The following key words were used: “randomized trial,” “registry,” “MI,” “STEMI,” “reperfusion,” “primary angioplasty,” “IIb/IIIa inhibitors,” “abciximab,” “tirofiban,” and “eptifibatide.”
Two investigators assessed the following eligibility criteria: (1) well-defined STEMI definition, i.e., clear-cut electrocardiographic signs on admission, (2) within each study, comparison of tirofiban or eptifibatide in the active group to ABX in the control group, and (3) reporting of the incidence of the composite end point of “all-cause” death plus nonfatal reinfarction. Data were independently extracted by 2 investigators. Where possible, authors were contacted to provide additional unpublished data. Disagreements were resolved by consensus.
The primary end point was a composite of all-cause death and nonfatal reinfarction up to 30 days of follow-up. Consistently, nonfatal reinfarction was defined as the recurrence of typical symptoms with new ST-segment elevation and/or new elevation of creatine kinase-MB >2 times the upper reference limit or a further increase >50% above the previous lowest level in patients with already increased enzyme levels, except for a few studies. Secondary end points were (1) short-term (up to 30 days) death from all causes and nonfatal reinfarction analyzed separately, (2) long-term (up to 12 months) all-cause death, and (3) rate of major bleeding during the hospital stay, as defined in each study. Angiographic outcomes were initial and final Thrombolysis In Myocardial Infarction grade 3 flows. Information about ST-segment resolution was also assessed. Because studies evaluated different ST-segment resolution criteria, with mixed use of >70% and 50% cutoffs, we analyzed ST-segment resolution with the most stringent >70% cutoff, which is linked to the lowest mortality rate in reperfused STEMI.
Because ABX-treated patients showed an average event (short-term death and nonfatal reinfarction) rate of 5% with an SD of 1.5% across studies, to demonstrate SM noninferiority we selected a prespecified noninferiority margin corresponding to the ABX SD value. Using such an assumption, when the sample in each group is 2,600, a 2-group large-sample normal approximation test of proportions with a 1-sided 0.05 significance level has 80% power to reject the null hypothesis that SM and ABX are not equivalent (difference in proportions, π SM − π ABX , is 0.015 or further from 0 in the same direction) in favor of the alternative hypothesis that the proportions in the 2 groups are equivalent, assuming that the expected difference in proportions is 0.0 and the proportion in the ABX group is 5% (nQuery Advisor 6.0, Statistical Solutions, Ltd., Cork, Ireland).
Statistical analyses were performed using Comprehensive Meta-Analysis 2.2 (Bio-Stat Solutions, Englewood, New Jersey) and SPSS 13.0 (SPSS, Inc., Chicago, Illinois). Two prespecified sensitivity analyses were performed: (1) according to type of studies, i.e., RCTs versus registries; and (2) according to treatment of interest, i.e., tirofiban or eptifibatide, to detect differences, if any, from ABX.
Chi-square test and Fisher’s exact test, if appropriate, were used to compare SM to ABX treatment for all outcomes to assess differences between proportions with calculations of odd ratios (ORs) and 95% confidence intervals (CIs). The OR was calculated with a fixed- or random-effect model, reporting the former due to a lack of significant heterogeneity (by Q statistic, p <0.10), quantified with an I 2 value (low ≤25%, high ≥75%). The p-value threshold was set at 0.05 for effect size.
Funnel plots were used to assess for the presence of publication bias by plotting the precision (inverse of the SE) against the log OR ratio. The trim-and-fill method was used to obtain symmetry in the funnel plot and to determine the impact of hypothetical negative or imputed studies on pooled estimates.
Our study was performed in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines.
Results
Among 875 potentially relevant reports, we identified 6 RCTs and 4 registries suitable for analysis, 8 published as regular publications, 1 in abstract form, and 1 presented at the American Heart Association 2007 scientific sessions. Tirofiban was used in 5 RCTs, and eptifibatide in 1 RCT and 4 registries. Criteria for STEMI diagnosis were uniform across all studies, i.e., chest pain >30 minutes with ST-segment elevation of ≥0.1 mV in ≥2 contiguous leads on admission electrocardiogram or new-onset left bundle branch block. Long-term follow-up was available in 2 RCTs and 3 registries. All together, 7,349 patients were evaluated, 4,653 treated with SM (919 received tirofiban, 3,734 eptifibatide), and 2,696 with ABX. All studies provided a periprocedural glycoprotein IIb/IIIa receptor antagonist, except 3 trials, allowing also preprocedural administration. The population appeared to be homogenous in contemporary PPCI (routine coronary stenting in all studies) and in age, male gender, coronary risk factors, hemodynamic status, and severity of coronary artery disease ( Table 1 ). Only previous MI and previous revascularization were more frequently reported among SM treatments, due to registry data, because no differences were detected between SM and ABX treatments among RCTs (7.8% vs 6.4%, p = 0.28, and 6.0% vs 5.9%, p = 0.95, respectively).
| Variable | SM | ABX | p Value |
|---|---|---|---|
| Age (years) | 61.2 ± 12.7 | 61.8 ± 12.2 | 0.22 |
| Men | 3,258/4,500 (72%) | 1,692/2,296 (74%) | 0.48 |
| Hypertension | 2,585/4,274 (60%) | 1,170/2,095 (56%) | 0.08 |
| Smoker | 1,827/3,980 (46%) | 923/1,937 (48%) | 0.46 |
| Hyperlipidemia | 559/974 (57%) | 458/887 (51%) | 0.19 |
| Diabetes | 873/4,500 (19%) | 445/2,166 (21%) | 0.39 |
| Killip class ≥2 | 524/4,391 (12%) | 291/2,216 (13%) | 0.23 |
| Previous myocardial infarction | 949/4,450 (22%) | 543/2,246 (24%) | 0.04 |
| Previous revascularization | 909/3,980 (23%) | 319/1,937 (16%) | 0.0001 |
| 1-Vessel disease | 1,994/3,921 (51%) | 931/1,907 (49%) | 0.41 |
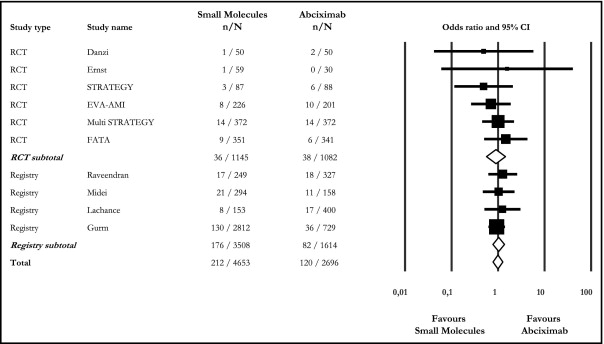
Overall, the short-term rate of death and nonfatal reinfarction (i.e., primary efficacy end point for which study power was calculated) was similar between SM and ABX treatments (4.6% vs 4.5%, OR 0.99, 95% CI 0.78 to 1.27), with an absolute difference of 0.1% (95% CI −1.06 to 0.8; Figure 1 ). Because the equivalence limit was set at ±1.5%, and because the upper point estimate (0.8%) of the CIs did not cross the prespecified limit, the noninferiority of SM treatment was documented. Similarly, there were no differences between RCTs (3.1% vs 3.5%, OR 0.90, 95% CI 0.56 to 1.44, I 2 0.0%) and registries (5.0% vs 5.1%, OR 1.03, 95% CI 0.77 to 1.37, I 2 0.0%) when separately analyzed ( Figure 1 ).
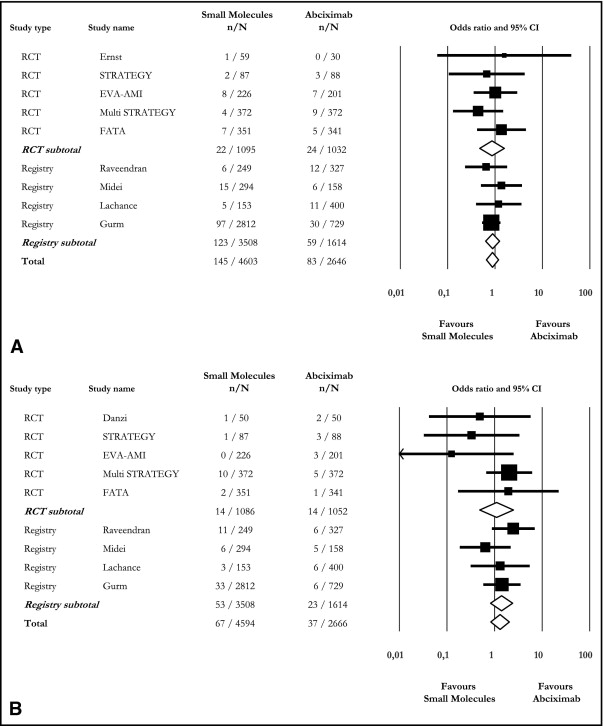
Rates of short-term death (3.2% vs 3.1%, OR 0.88, 95% CI 0.66 to 1.19) and nonfatal reinfarction (1.5% vs 1.4%, OR 1.31, 95% CI 0.84 to 2.04) were similar between SM and ABX treatments, and this applied to RCTs and registries ( Figure 2 ). Long-term death was also not different (4.8% vs 5.4%, OR 0.83, 95% CI 0.58 to 1.19, I 2 4%) because 56 of 1,155 treated with SM and 73 of 1,345 patients treated with ABX had died over the long run.
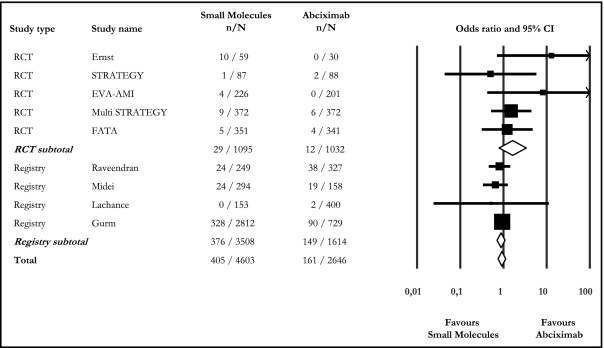
Major bleeding was reported in 9 of 10 studies. Overall, SM and ABX treatments did not differ (8.8% vs 6.1%, OR 0.92, 95% CI 0.75 to 1.13), and this was also documented among RCTs and registries ( Figure 3 ).
Baseline rate of Thrombolysis In Myocardial Infarction grade 3 flow (OR 1.05, 95% CI 0.85 to 1.31, I 2 0.0%) and postprocedural Thrombolysis In Myocardial Infarction grade 3 flow (OR 0.84, 95% CI 0.69 to 1.02, I 2 0.0%) were similar between SM and ABX treatments. RCTs and registries did not differ when separately analyzed ( Table 2 ).
| Initial TIMI Grade 3 Flow | p Value | Final TIMI Grade 3 Flow | p Value | |||
|---|---|---|---|---|---|---|
| SM | ABX | SM | ABX | |||
| Randomized clinical trials | ||||||
| Danzi et al | N/A | N/A | 44/50 (88.0%) | 43/50 (86.0%) | 0.77 | |
| Ernst et al | N/A | N/A | 49/57 (86%) | 26/28 (93%) | 0.36 | |
| STRATEGY | 13/87 (14.9%) | 10/88 (11.4%) | 0.49 | 84/87 (96.6%) | 82/88 (93.2%) | 0.32 |
| EVA-AMI | 76/226 (33.6%) | 64/201 (31.8%) | 1.04 | 186/226 (82.3%) | 171/201 (85.1%) | 0.44 |
| MULTISTRATEGY | 75/372 (20.2%) | 82/372 (22.0%) | 0.53 | 338/372 (90.1%) | 346/372 (93.0%) | 0.28 |
| FATA | 58/351 (16.5%) | 48/341 (14.1%) | 0.37 | 318/351 (90.6%) | 304/341 (89.1%) | 0.53 |
| Subtotal | 222/1,036 (21.4%) | 204/1,002 (20.4%) | 0.62 | 1,019/1,143 (89.1%) | 972/1,080 (89.9%) | 0.61 |
| Registries | ||||||
| Raveendran et al | N/A | N/A | 220/249 (88.4%) | 300/327 (91.7%) | 0.18 | |
| Gurm et al | N/A | N/A | 2,575/2,812 (91.5%) | 680/729 (93.3%) | 0.13 | |
| Midei et al | N/A | N/A | 291/294 (98.9%) | 156/158 (98.7%) | 0.81 | |
| Lachance et al | N/A | N/A | N/A | N/A | ||
| Subtotal | N/A | N/A | 3,086/3,355 (91.9%) | 1,136/1,214 (93.6%) | 0.06 | |
| Total | 222/1,036 (21.4%) | 204/1,002 (20.4%) | 0.62 | 4,105/4,498 (91.2%) | 2,108/2,294 (91.9%) | 0.08 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


