The relation between hepatic serum markers within the normal range and cardiovascular risk is uncertain. We sought to address this issue within a prospective randomized trial of statin therapy. Men and women (n = 17,515) free of cardiovascular disease participating in a randomized placebo controlled trial of rosuvastatin 20 mg/day had baseline levels of alanine aminotransferase (ALT) below <40 IU/l and were followed prospectively for the first-ever cardiovascular events. Cox proportional hazards models were used to calculate the relative risks of these events according to increasing tertiles and each SD increase in baseline ALT levels. ALT levels at study entry, all within the normal range, were inversely associated with age, smoking status, and inflammation and were positively associated with male gender, alcohol use, and triglycerides. Incident cardiovascular event rates were highest in those in the lowest tertile of baseline ALT; specifically, incidence rates were 1.43, 0.98, and 0.85 per 100 person-years of exposure for those in the lowest, middle, and highest tertile of baseline ALT within the normal range, respectively (p <0.001). These inverse effects remained statistically significant after multivariate adjustment for a wide range of vascular risk factors risk markers such that each higher SD unit of ALT was associated with an 18% lower event rate (relative risk 0.82, 95% confidence interval 0.72 to 0.93, p = 0.002). The efficacy of statin therapy was not modified by baseline ALT level. In conclusion, increasing ALT levels within the normal range are inversely associated with future cardiovascular risk but had limited clinical utility and also did not modify the efficacy of statin therapy.
Graphical Abstract
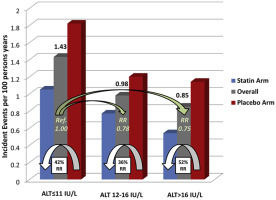
Cardiovascular events incidence according to ALT tertiles and treatment arm. Green arrows displaying the overall RR of intermediate and higher ALT tertiles versus lower ALT tertile. White arrows displaying the relative risk reduction of statin therapy versus placebo within each tertile.
Alanine aminotransferase (ALT) is released by the liver both in pathologic and normal physiological conditions. Clinically, levels of ALT above the upper limit of normality have been associated with increased risks for hepatic and cardiovascular events, in part due to underlying nonalcoholic fatty liver disease (NAFLD) and its consequent effects on metabolic dysfunction and diabetes. Besides, statin therapy can increase ALT levels and elevations over 3 times the upper normal limit of ALT have in turn been considered a relative contraindication for therapy with this class of lipid-lowering agent. In contrast, recent data have suggested an apparent paradox between ALT levels within the normal range, incident vascular event rates, and the efficacy of statin therapy. In particular, prospective cohort studies have reported inverse relations between ALT levels within the normal range and subsequent risks for cardiovascular events and all-cause mortality such that those in higher ALT stratum have reduced event rates versus those in the lower ALT group. Furthermore, reports from 2 statin trials have suggested that those with ALT levels higher than the upper limit of normal obtain a greater relative risk reduction from lipid-lowering therapy versus those with normal ALT levels. Whether the efficacy of statin therapy is modified by ALT levels within the normal range, however, is uncertain. We addressed these issues in the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, a contemporary randomized double-blind placebo-controlled evaluation of rosuvastatin 20 mg. That trial included 17,515 apparently healthy men and women with ALT levels ≤40 IU/l who were followed prospectively for future vascular events.
Methods
The sample was derived from JUPITER ( ClinicalTrial.gov NCT00239681 ), a randomized, double-blind, placebo-controlled trial designed to investigate whether rosuvastatin 20 mg/day compared to placebo decreases the rate of the first-ever cardiovascular events in apparently healthy men aged >50 years and women >60 years with low-density lipoprotein (LDL) cholesterol <130 mg/dl who were at increased vascular risk because of a high-sensitivity C reactive protein (hsCRP) level ≥2 mg/l. The full details of the trial protocol have been previously presented. The trial exclusion criteria included diabetes, treatment with any lipid-lowering therapy in the 6 weeks preceding randomization, current use of postmenopausal hormonal replacement therapy, previously known ALT levels higher than 2 times the upper limit of normal, creatinine >2.0 mg/dl, uncontrolled hypertension or hypothyroidism, a history of malignancy within 5 years, or another serious medical condition that might compromise patient safety or successful completion of the study.
Of 17,783 participants enrolled in this trial, 268 had baseline ALT levels >40 IU/l, the upper normal limit cutoff in the trial. Thus, the present analysis included 17,515 participants with baseline ALT levels within the study normal limits. Differences in clinical characteristics and 10-year predicted risk Framingham-based ATP-III risk estimator and Reynolds risk score between those in each ALT tertile were tested for trend by linear regression if normal and Jonckheere-Terpstra for nonparametric or categorical variables. We displayed the age- and gender-adjusted cumulative incident cardiovascular events curves for ALT tertiles and the age- and gender-adjusted hazard ratios (HRs) of the second and third tertiles versus the first. The relation of baseline ALT levels with future vascular events was addressed by its tertiles and SD units in Cox proportional hazards models to calculate crude and adjusted incidence rates for the composite end point of myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina pectoris, or death from cardiovascular causes. Models were adjusted for age, gender, waist circumference, smoking status, alcohol consumption, systolic blood pressure, fasting glucose, plasma lipid levels, statin treatment, and hsCRP. Effect modification was addressed by stratified analyses according to age categories (<65 and ≥65 years), gender, smoking status, waist circumference categories (normal ≤102 cm for men and ≤88 cm for women, and abnormal otherwise), alcohol consumption categories (no or infrequent use if less than weekly consumption and regular otherwise), metabolic syndrome, and Reynolds risk score (<7.5% and ≥7.5%) categories. Rosuvastatin relative risk reductions were compared to placebo for each ALT tertile to evaluate effect modification. We also displayed the age- and gender-adjusted cumulative incident cardiovascular events curves for treatment arms within each ALT tertile and the age- and gender-adjusted HRs for rosuvastatin versus placebo. To address the HR for events across the ALT range in a more flexible way, we plotted adjusted smoothed spline with ALT knots at the 5, 25, 50, 75, and 95 percentiles values.
Cardiovascular risk prediction based on Reynolds risk score was compared in models with and without the addition of ALT in nonstatin users. The Reynolds risk score predicts cardiovascular death, myocardial infarction, stroke, and coronary revascularization. We compared C -statistics for the 2 models and computed the integrated discrimination improvement (IDI) and relative IDI and categorical net reclassification improvement (NRI). The group cutoffs for NRI were 1% and 1.5%, which correspond to 5% and 7.5% risk in 10 years of follow-up assuming events accumulate linearly every 2 years.
JUPITER was an investigator-initiated trial. The sponsor of the study collected the trial data and monitored the study sites but had no access to unblinded data until after the drafting of the trial primary report. All statistical analyses were done by the investigators and the academic study statistician. Trial principal investigator (P.M.R.) had full access to all study data and had final responsibility for the decision to submit these data for publication.
Results
JUPITER trial participants in the lowest tertile of ALT (≤11 IU/l) were significantly older than those in the highest tertile (>16 IU/l), were more likely to smoke, had moderately higher levels of hsCRP, and a higher Reynolds risk score ( Table 1 ). In contrast, those in the highest tertile of ALT at baseline were more likely to be men, had more prevalent daily alcohol consumption, higher body mass index, and higher levels of triglycerides. Despite younger age, those in the highest baseline tertile of ALT had a significantly greater prevalence of metabolic syndrome, had more coronary heart disease risk factors, and higher Framingham risk score. Finally, the LDL reduction after 1 year was similar across the tertiles.
| ALT Range (IU/L) | Lower | Intermediate | Higher | p value |
|---|---|---|---|---|
| ≤11 N = 5505 | 12-16 N = 5896 | >16 N = 6114 | ||
| Age (years) | 68.8 (7.7) | 66.4 (7.4) | 63.6 (7.1) | <0.001 |
| Men | 48.2% | 60.5% | 74.6% | <0.001 |
| Body mass index (kg/m 2 ) | 27.9 (6.0) | 28.9 (5.4) | 30.0 (5.7) | <0.001 |
| Waist Circumference (cm) | 95.5 (13.6) | 98.7 (14.1) | 102.3 (13.2) | <0.001 |
| Alcohol ≥1 drink per day | 13.3% | 18.3% | 23.4% | <0.001 |
| Current Smoker | 18.6% | 15.0% | 14.1% | <0.001 |
| Estimated Glomerular Filtration Rate (ml/min) | 74.3 (18.8) | 74.9 (17.1) | 76.6 (16.3) | <0.001 |
| Family History of Early Coronary Heart Disease | 10.8% | 11.6% | 12.2% | 0.068 |
| Metabolic Syndrome | 33.9% | 39.5% | 50.5% | <0.001 |
| Hypertension | 57.2% | 56.6% | 57.9% | 0.361 |
| Systolic Blood Pressure (mmHg) | 135 (17) | 136 (17) | 136 (17) | <0.001 |
| Diastolic Blood Pressure (mmHg) | 80 (9) | 81 (9) | 82 (9) | <0.001 |
| Fasting Glucose (mg/dl) | 92.7 (11.1) | 94.7 (11.5) | 96.8 (11.9) | <0.001 |
| HbA1C (%) | 5.7 (0.5) | 5.7 (0.4) | 5.7 (0.4) | 0.200 |
| Total Cholesterol (mg/dl) | 182 (25) | 184 (24) | 184 (24) | <0.001 |
| HDL Cholesterol (mg/dl) | 53.3 (15.6) | 51.7 (15.0) | 49.2 (14.9) | <0.001 |
| LDL Cholesterol (mg/dl) | 104 (19) | 105 (18) | 104 (19) | 0.748 |
| Triglycerides (mg/dl)* | 107 (80 – 149) | 117 (84 – 166) | 132 (93 – 193) | <0.001 |
| Apolipoprotein-a (mg/dl) | 167 (31) | 166 (31) | 164 (30) | <0.001 |
| Apolipoprotein-b (mg/dl) | 105 (21) | 109 (21) | 112 (22) | <0.001 |
| High sensitive C Reactive Protein (mg/L)* | 4.7 (3.0 – 8.3) | 4.2 (2.8 – 6.8) | 4.1 (2.8 – 6.5) | <0.001 |
| Framingham Risk Score* | 10 (5 – 16) | 10 (6 – 16) | 11 (6 – 16) | <0.001 |
| Reynolds Risk Score* | 10.0 (5.5 – 17.3) | 9.6 (5.6 – 16.1) | 9.4 (5.9 – 15.5) | 0.009 |
| Drug (Rosuvastatin) | 50.0% | 50.2% | 49.6% | 0.801 |
| Delta LDL Cholesterol (mg/dL) | -14 (-49 – +6) | -12 (-51 – +7) | -14 (-51 – +7) | 0.521 |
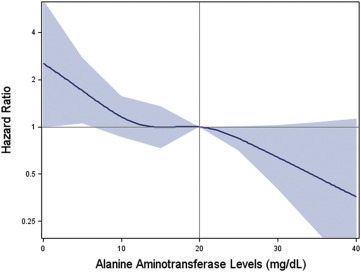
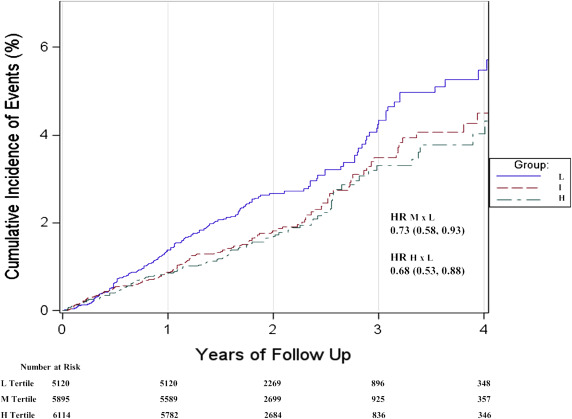
As shown in the age- and gender-adjusted data of Figure 1 , those in the lowest tertile of baseline ALT had the highest rates of future cardiovascular events during the 5-year follow-up period. Specifically, Table 2 reports incidence rates of 1.43, 0.98, and 0.85 per 100 person-years of exposure for those in the lower, intermediate, and higher tertile of baseline ALT within the normal range, respectively (p <0.001). These effects persisted after adjustment for age, smoking, gender, waist circumference, blood pressure, glucose, high-density lipoprotein cholesterol, triglycerides, alcohol use, statin treatment, and hsCRP such that each increasing SD of ALT was associated with an 18% multivariate adjusted decrease in risk (95% confidence interval [CI] 0.72 to 0.93, p = 0.002). The HR splines displayed in Figure 2 confirms the monotonic decreasing risk of vascular events with increasing ALT values within the normal ALT range. As reported in Table 1 , this direction of effect was concordant with projected Reynolds but opposite to Framingham Risk Scores for this cohort. Effects were consistent in all clinical strata evaluated ( Table 3 ).
| ALT Range (IU/L) | Lower Tertile | Intermediate Tertile | Higher Tertile | p value for trend | HR per 1-SD Increase | p value |
|---|---|---|---|---|---|---|
| ≤11 (N=5505) | 12-16 (N=5896) | >16 (N=6114) | ||||
| Primary Events (%) | 163 (3.0%) | 122 (2.1%) | 107 (1.8%) | |||
| Cardiovascular Death (%) | 33 (0.6%) | 15 (0.3%) | 16 (0.3%) | |||
| Stroke (%) | 54 (1.0%) | 22 (0.4%) | 19 (0.3%) | |||
| Myocardial Infarction (%) | 33 (0.6%) | 38 (0.6%) | 25 (0.4%) | |||
| Hosp. Unstable Angina (%) | 15 (0.3%) | 10 (0.2%) | 17 (0.3%) | |||
| Arterial Revascularization (%) | 46 (0.8%) | 55 (0.9%) | 45 (0.7%) | |||
| Primary Events/100 person years | 1.43 | 0.98 | 0.85 | |||
| HR Primary Event (Model 1) | Ref. | 0.73 (0.58 – 0.93) | 0.68 (0.53 – 0.88) | 0.003 | 0.78 (0.69 – 0.89) | <0.001 |
| HR Primary Event (Model 2) | Ref. | 0.77 (0.60 – 0.98) | 0.73 (0.56 – 0.96) | 0.017 | 0.81 (0.72 – 0.92) | 0.001 |
| HR Primary Event (Model 3) | Ref. | 0.78 (0.61 – 0.99) | 0.74 (0.57 – 0.97) | 0.025 | 0.82 (0.72 – 0.93) | 0.002 |