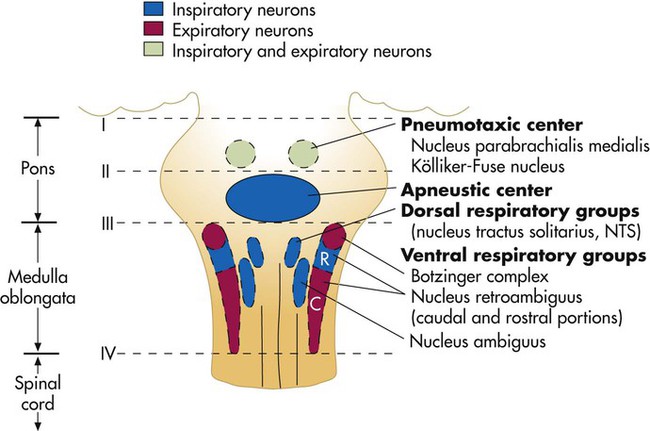
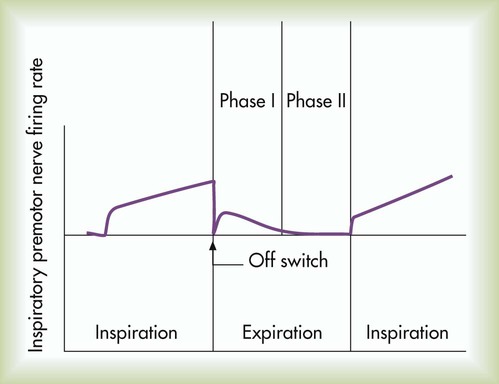
After reading this chapter you will be able to: Animal experiments show that transecting the brainstem just below the medulla (Figure 14-1, level IV) stops all ventilatory activity. However, breathing continues rhythmically after the brainstem is transected just above the pons (see Figure 14-1, level I). Until more recently, physiologists thought that separate inspiratory and expiratory neuron “centers” in the medulla were responsible for the cyclic pattern of breathing. Researchers believed that inspiratory and expiratory neurons fired by self-excitation and that they mutually inhibited one another. More recent evidence shows that inspiratory and expiratory neurons are anatomically intermingled and do not inhibit one another.1 No clearly separate inspiratory and expiratory centers exist. Instead, the medulla contains several widely dispersed respiratory-related neurons, as shown in Figure 14-1. The dorsal respiratory groups (DRGs) contain mainly inspiratory neurons, whereas the ventral respiratory groups (VRGs) contain both inspiratory and expiratory neurons. As shown in Figure 14-1, DRG neurons are mainly inspiratory neurons located bilaterally in the medulla. These neurons send impulses to the motor nerves of the diaphragm and external intercostal muscles, providing the main inspiratory stimulus.1 Many DRG nerves extend into the VRGs, but few VRG fibers extend into the DRGs. Reciprocal inhibition is an unlikely explanation for rhythmic, spontaneous breathing.1 VRG neurons are located bilaterally in the medulla in two different nuclei and contain inspiratory and expiratory neurons (see Figure 14-1). Some inspiratory VRG neurons send motor impulses through the vagus nerve to the laryngeal and pharyngeal muscles, abducting the vocal cords and increasing the diameter of the glottis. Other VRG inspiratory neurons transmit impulses to the diaphragm and external intercostal muscles. Still other VRG neurons have mostly expiratory discharge patterns and send impulses to the internal intercostal and abdominal expiratory muscles. The exact origin of the basic rhythmic pattern of ventilation is unknown. No single group of pacemaker cells has been identified. Two predominant theories of rhythm generation are the pacemaker hypothesis and the network hypothesis.2 The pacemaker hypothesis holds that certain medullary cells have intrinsic pacemaker properties (i.e., rhythmic self-exciting characteristics) and that these cells drive other medullary neurons. The network hypothesis suggests that rhythmic breathing is the result of a particular pattern of interconnections between neurons dispersed throughout the rostral VRG, the pre-Bötzinger complex, and the Bötzinger complex. This hypothesis assumes that certain populations of inspiratory and expiratory neurons inhibit one another and that one of the neuron types fires in a self-limiting way, such that it becomes less responsive the longer it fires. There is no definitive proof of either hypothesis; the precise origin of respiratory rhythm generation remains elusive.2 The inspiratory muscles do not receive an instantaneous burst of signals from the dorsal and ventral inspiratory neurons. Rather, the firing rate of DRG and VRG inspiratory neurons increases gradually at the end of the expiratory phase, creating a ramp signal (Figure 14-2). The inspiratory muscles contract steadily and smoothly, gradually expanding the lungs rather than filling them in an abrupt inspiratory gasp. During exercise, various reflexes and receptors influence the medullary neurons, steepening the ramp signal and filling the lungs more rapidly. During quiet breathing, inspiratory neurons fire with increasing frequency for approximately 2 seconds and then abruptly switch off, allowing expiration to proceed for approximately 3 seconds.3 At the start of expiration, inspiratory neurons again fire briefly, retarding the early phase of expiration (see Figure 14-2). The inhibitory neurons that switch off the inspiratory ramp signal are controlled by the pneumotaxic center and pulmonary stretch receptors, which are discussed later in this chapter. If the brainstem is transected above the medulla (see Figure 14-1, level III), spontaneous respiration continues, although in a more irregular pattern. The pons does not promote rhythmic breathing; rather, it modifies the output of the medullary centers. Figure 14-1 shows two groups of neurons in the pons: (1) the apneustic center and (2) the pneumotaxic center. The pneumotaxic center is a bilateral group of neurons located in the upper pons (see Figure 14-1). The pneumotaxic center controls the “switch-off” point of the inspiratory ramp, controlling inspiratory time. Strong pneumotaxic signals increase the respiratory rate, and weak signals prolong inspiration and increase tidal volumes. The exact nature of the interaction between the pneumotaxic and apneustic centers is poorly understood. They apparently work together to control the depth of inspiration.3 The Hering-Breuer inflation reflex, described by Hering and Breuer in 1868, is generated by stretch receptors located in the smooth muscle of both large and small airways. When lung inflation stretches these receptors, they send inhibitory impulses through the vagus nerve to the DRG neurons, stopping further inspiration. In this way, the Hering-Breuer reflex has an effect similar to that of the pneumotaxic center. In adults, the Hering-Breuer reflex is activated only at large tidal volumes (≥800 to 1000 ml) and apparently is not an important control mechanism in quiet breathing.2 This reflex is important, however, in regulating respiratory rate and depth during moderate to strenuous exercise. Sudden collapse of the lung stimulates strong inspiratory effort. This inspiratory effort may be the result of decreased stretch receptor activity, or it may be caused by the stimulation of other receptors, such as the irritant receptors and J-receptors (discussed later). Although it is unclear which receptors are involved, it is clear that the vagus nerve is the pathway (as it is for the Hering-Breuer reflex) and that the effect is hyperpnea.1 The deflation reflex is probably responsible for the hyperpnea observed with pneumothorax (air in the pleural space). In 1889, Head observed that if the Hering-Breuer reflex is blocked by cooling the vagus nerve, lung hyperinflation causes a further increase in inspiratory effort—the opposite of the Hering-Breuer reflex. The receptors for this reflex are called rapidly adapting receptors because they stop firing promptly after a volume change occurs. The Head reflex may help maintain large tidal volumes during exercise and may be involved in periodic deep sighs during quiet breathing. Periodic sighs help prevent alveolar collapse, or atelectasis. The Head reflex also may be responsible for the first breaths of a newborn.1 Proprioceptors in muscles, tendons, and joints and pain receptors in muscles and skin send stimulatory signals to the medullary respiratory center. Such stimuli increase medullary inspiratory activity and cause hyperpnea.4 For this reason, moving the limbs, slapping or splashing cold water on the skin, and other painful stimuli stimulate ventilation in patients with respiratory depression. Proprioceptors in joints and tendons may be important in initiating and maintaining increased ventilation at the beginning of exercise. Passive limb movement around a joint increases breathing rate in both anesthetized animals and unanesthetized humans.4 Muscle spindles in the diaphragm and intercostal muscles are part of a reflex arc that helps the muscles adjust to an increased load. Muscle spindles are sensing elements located on intrafusal muscle fibers, arranged parallel to the main extrafusal muscle fibers (Figure 14-3). The extrafusal fibers that elevate the ribs are innervated by different motor fibers (alpha fibers) than the fibers that innervate the intrafusal spindle fibers (gamma fibers). When the main extrafusal muscle fiber and the intrafusal fibers contract simultaneously, the sensing element (spindle) of the intrafusal muscle fiber stretches and sends impulses over spindle afferent nerves directly to the spinal cord (see Figure 14-3
Regulation of Breathing
 Identify where the structures that regulate breathing are located.
Identify where the structures that regulate breathing are located.
 Describe how the inspiratory and expiratory neurons in the medulla establish the basic pattern of breathing.
Describe how the inspiratory and expiratory neurons in the medulla establish the basic pattern of breathing.
 Describe the effect that impulses from the pneumotaxic and apneustic centers in the pons have on the medullary centers of breathing.
Describe the effect that impulses from the pneumotaxic and apneustic centers in the pons have on the medullary centers of breathing.
 Identify the effect of various reflexes on breathing.
Identify the effect of various reflexes on breathing.
 Describe how the central and peripheral chemoreceptors differ in the way they regulate breathing.
Describe how the central and peripheral chemoreceptors differ in the way they regulate breathing.
 State how the central chemoreceptors respond differently to respiratory and nonrespiratory acid-base disorders.
State how the central chemoreceptors respond differently to respiratory and nonrespiratory acid-base disorders.
 Describe how the regulation of breathing in individuals with chronic hypercapnia differs from the regulation of breathing in healthy individuals.
Describe how the regulation of breathing in individuals with chronic hypercapnia differs from the regulation of breathing in healthy individuals.
 Describe why administering oxygen to patients with chronic hypercapnia poses a special risk that is not present in healthy individuals.
Describe why administering oxygen to patients with chronic hypercapnia poses a special risk that is not present in healthy individuals.
 Describe why ascending to a high altitude has different immediate and long-term effects on ventilation.
Describe why ascending to a high altitude has different immediate and long-term effects on ventilation.
 State why mechanically ventilated patients with head injuries may benefit from deliberate hyperventilation.
State why mechanically ventilated patients with head injuries may benefit from deliberate hyperventilation.
 Describe the characteristics of abnormal breathing patterns.
Describe the characteristics of abnormal breathing patterns.
Medullary Respiratory Center
Dorsal Respiratory Groups
Ventral Respiratory Groups
Inspiratory Ramp Signal
Pontine Respiratory Centers
Pneumotaxic Center
Reflex Control Of Breathing
Hering-Breuer Inflation Reflex
Deflation Reflex
Head Paradoxical Reflex
Peripheral Proprioceptors
Muscle Spindles
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Regulation of Breathing