X-ray radiation exposure is of great concern for patients undergoing structural heart interventions. In addition, a larger group of medical staff is required and exposed to radiation compared with percutaneous coronary interventions. This study aimed at quantifying radiation dose reduction with implementation of specific image noise reduction technology (NRT) in transcatheter aortic valve implantation (TAVI) procedures. We retrospectively analyzed 104 consecutive patients with TAVI procedures, 52 patients before and 52 after optimization of x-ray radiation chain, and implementation of NRT. Patients with 1-step TAVI and complex coronary intervention, or complex TAVI procedures, were excluded. Before the procedure, all patients received a multislice computed tomography scan, which was used to size aortic annulus, select the optimal implantation plane, valve type and size, and guide valve implantation using a software tool. Air kerma and kerma–area product were compared in both groups to determine patient radiation dose reduction. Baseline parameters, co-morbidity, or procedural data were comparable between groups. Mean kerma–area product was significantly lower (p <0.001) in the NRT group compared with the standard group (60 ± 39 vs 203 ± 106 Gy × cm 2 , p <0.001), which corresponds to a reduction of 70%. Mean air kerma was reduced by 64% (494 ± 360 vs 1,355 ± 657 mGy, p <0.001). In conclusion, using optimized x-ray chain combined with specific image noise reduction technology has the potential to significantly reduce by 2/3 radiation dose in standard TAVI procedures without worsening image quality or prolonging procedure time.
Unlike percutaneous coronary interventions, exposure to radiation in transcatheter aortic valve implantation (TAVI) procedures involves not only patient and operator but also additional personnel taking care of the patient. Furthermore, some of the additional personnel, such as anesthetist, cardiac surgeon, echocardiographer, and nurses, might work in a zone around the x-ray radiation source and cannot be fully protected against radiation exposure by (lead) shielding. Current, mandatory, measures to reduce radiation exposure include using state of the art personal protection devices and using radiation in a dose as low as reasonably achievable (the ALARA principle). However, it would be even better to have a technology that uses less radiation while maintaining or improving image quality. The ClarityIQ imaging technology (Philips Healthcare, Best, The Netherlands) implements an x-ray image acquisition chain optimization with image noise reduction technology (NRT) and helps to reduce radiation exposure while maintaining image quality. The aim of our study was to research to what extent the implementation of this new technology would reduce radiation exposure under the highly standardized setting of TAVI procedures.
Methods
We retrospectively analyzed 104 consecutive patients, 52 before and 52 patients after implementation of NRT to our x-ray system. Patients with 1-stage complex percutaneous coronary intervention (bifurcations, >1 vessel involved) and TAVI procedure and patients with complex TAVI procedures (>1 valve) were excluded. Patients who required covered stent implantation into the femoral artery because of insufficient closure of the puncture site despite the use of a closure device were not excluded, although placement of these stents prolonged procedure time and mandated more radiation. Only radiation exposure data from the TAVI procedure itself including 1-stage coronary interventions and femoral artery stenting for closure of TAVI access site were collected for this study. Badge dosimetry from operators was not reported because badges were not exclusively worn during TAVI procedures. Fluoroscopy time, procedure time, air kerma, and kerma–area product (KAP) were recorded. Air kerma represents the energy extracted from an x-ray beam per unit mass of air in a small irradiated air volume. KAP is calculated by integration of air kerma across the entire x-ray beam emitted from the x-ray tube. KAP, previously called dose–area product, is a surrogate for the amount of radiation energy delivered to the patient.
Basic demographic data, transthoracic and transesophageal echocardiography data, information about coronary artery disease from preprocedural coronary angiograms, and contrast computed tomography (CT) scans were extracted from patient records. Body surface area was calculated using the Dubois formula. At our center, a contrast multislice CT scan is routinely made in preparation for TAVI procedures as previously described. The CT scan is used to plan access route (transfemoral, subclavian, transapical) and to determine vessel size, calcification, and its distribution in main vessels. Furthermore, the CT scan is routinely imported into the HeartNavigator tool (Philips Healthcare, DA Best, The Netherlands, and Andover, Massachusetts). This tool, which was cleared by the US Food and Drug Administration in December 2011, allows for accurate sizing of the aortic annulus, for choosing an optimal implantation plane and for virtual implantation of transcatheter valves of various valve types and sizes. Furthermore, the HeartNavigator combines CT data sets and real-time fluoroscopy imaging and helps to save both, radiation and contrast agent, by providing an overlay projection of the aortic root outline, aortic annulus, and coronary ostia onto the fluoroscopy screen during the procedure.
The TAVI program at our center started early in 2010. From the year thereafter, 150 to 180 procedures were carried out annually. All TAVI procedures within this study were carried out by the same operators, who had repeatedly completed full radiation safety training as required by German law. TAVI operators had an experience of >500 successful procedures before beginning of this study. All TAVI procedures were carried out following a standardized local TAVI protocol in all patients with no protocol changes during the study period. During the procedure, 7.5 fps were used for valve implantation and femoral artery stenting and 15 fps for coronary stent procedures.
Before implementation of specific NRT, the x-ray system used was a standard monoplane AlluraXper FD20 (Philips Healthcare, Best, The Netherlands). This is referred to as “standard” x-ray system throughout the manuscript. The AlluraXper FD20 was updated with NRT (ClarityIQ; Philips Healthcare) in November 2013. According to information provided by the manufacturer, the Philips Allura Clarity system with ClarityIQ technology consists of a novel x-ray imaging technology that combines noise reduction algorithms with state-of-the-art hardware to enable real-time image processing and to reduce patient entrance dose significantly. This is realized by optimization of the full image-processing chain including grid switch, pulse width, focus spot size, beam filtering, detector, and image processing engine, for every anatomic area and clinical task individually.
Furthermore, the image quality could be improved using a smaller focal spot sizes and enabling shorter pulses. The implementation of a motion compensation algorithm enables alignment of moving objects before averaging and allows to average more consecutive images as compared with traditional temporal noise reduction filters and to reduce radiation dose requirement per frame. Furthermore, a spatial noise reduction algorithm has been developed that takes into account the random nature of noise to distinguish between useful clinical information and noise in a single image. If a pixel is determined as noise, it is averaged with surrounding pixels. Because of the high computational power of the ClarityIQ technology, a larger neighborhood of pixels can be used for averaging compared with traditional spatial noise filters even when working with 30 fps and therefore increasing the likelihood of maintaining the relevant clinical information in the image. The final step in the ClarityIQ image processing is the image enhancement process that reduces so-called “low-frequency areas” to better compensate overexposed and underexposed image regions and, at the same time, enhance high-frequency edges and contours to sharpen contrast.
For the fluoroscopy modes I, II, and III, the maximum patient entrance dose for fluoroscopy was reduced by 50%, 50%, and 10%, respectively. This is the same relative reduction over the entire patient thickness range. For the cine exposures, operators can choose from 3 cine acquisition settings, 50%, 75%, and no patient dose reduction compared with standard, and switch settings anytime during the procedure. As a consequence of the ClarityIQ Image processing, it was possible to increase the additional copper filtration from 0.1 to 0.4 mm independent from patient body mass index. Lower energy photons do not contribute to image quality as they cannot penetrate the patient’s body but add needless, and harmful, radiation dose to the patient. Additional copper filtration reduces these photons and hence patient radiation dose.
To assess image quality, cine sequences from all procedures were shown to interventional cardiologists unaware of the imaging technology used. The interventionalists were asked to assign to the 2 groups all the cine sequences in a blinded fashion.
Data in tables are presented as means ± standard deviation where indicated. Statistical analysis was performed with Sigma Stat (SPSS Science Inc., Chicago, Illinois). The Student t test was used to detect statistical significant differences between groups. For nonparametric values, a rank-sum test was applied. Statistical significance was accepted at p <0.05.
Results
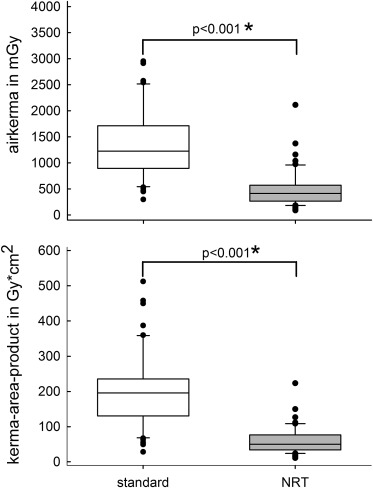
After implementation of the NRT, patient radiation dose could be reduced by at least 2/3 in our patients who underwent TAVI ( Figure 1 ). We did not find a significant loss in imaging quality. Interventional cardiologists shown cine sequences of the procedures, and being unaware of the imaging technology used, identified correctly on average only 50 ± 7% of sequences with NRT and 51 ± 6% of sequences with standard technology.
Baseline characteristics of standard and NRT group are summarized in Table 1 . Both groups did not differ significantly in age, gender, body weight, body mass index, and body surface area. Creatinine concentration was greater, and hence, GFRepi lower, in the NRT group compared with the standard group, although this did not reach statistical significance. Frailty, New York Heart Association class, and the commonly used risk scores EuroSCORE 2 and Society for Thoracic Surgeons score show a high risk for cardiac surgery in both groups. Patients in the NRT group tended to be sicker; however, there was no statistical significant difference between scores of both groups. When looking at cardiovascular risk factors and relevant co-morbidity, more patients in the NRT group had previous CABG and previous therapeutic chest radiation for cancer as compared with the standard group ( Table 2 ).
| Variable | Standard (n=52) | NRT (n=52) |
|---|---|---|
| Mean ± stdDev | Mean ± stdDev | |
| Age (years) | 80 ± 5 | 80 ± 7 |
| Females | 27 (52%) | 28 (54%) |
| body weight (kg) | 75 ± 17 | 76 ± 17 |
| Heigth (cm) | 165 ± 7 | 168 ± 9 |
| Body mass index (kg/m 2 ) | 27 ± 5 | 27 ± 5 |
| Body surface area (m 2 ) | 1.82 ± 0.20 | 1.84 ± 0.21 |
| Creatinine concentration (mg/dl) | 1.24 ± 0.46 | 1.42 ± 1.0 |
| GFRepi | 54 ± 19 | 50 ± 19 |
| Clinical frailty scale | 5.8 ± 0.8 | 6.1 ± 0.7 |
| NYHA functional class | 3.1 ± 0.6 | 3.2 ± 0.5 |
| Euroscore 2 | 13.5 ± 7.5 | 14.1 ± 8.8 |
| Society of Thoracic Surgeons’ risk model – mortality (%) | 8.2 ± 4.3 | 10.7 ± 7.0 |
| Society of Thoracic Surgeons‘ risk model – morbidity & mortality (%) | 34 ± 10 | 39 ± 14 |
| Standard (n=52) | NRT (n=52) | |
|---|---|---|
| Diabetes | 24 (46%) | 25 (48%) |
| Hypertension | 50 (96%) | 51 (98%) |
| Coronary artery disease | 29 (55%) | 26 (50%) |
| Previous coronary artery bypass grafting | 5 (10%) | 9 (17%) |
| Previous valve surgery | 1 (2%) | 2 (4%) |
| Atrial fibrillation | 22 (42%) | 25 (48%) |
| Chronic obstructive pulmonary disease | 15 (29%) | 11 (21%) |
| Prior chest radiation | 1 (2%) | 6 (12%) |
| Neurologic dysfunction | 16 (31%) | 15 (29%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


