Fig. 26.1
(a) Duplex ultrasound demonstrating moderate stenosis with an elevated peak systolic velocity of 200 cm/s in midportion of SFA stent. (b) Duplex ultrasound demonstrating high-grade stenosis with an elevated peak systolic velocity of 540 cm/s in proximal portion of SFA stent. (c) Angiogram demonstrating high-grade stenosis in proximal SFA stent. (d) Angiogram following atherectomy and re-angioplasty with resolution of in-stent stenosis
Failure of lower extremity endovascular interventions presents in a similar fashion to failed surgical bypasses. Claudicants will return to their pre-procedure walking tolerance, while patients with critical limb ischemia (CLI) and rest pain will likely return to rest pain. Certainly, claudicants may complain of decreasing walking tolerance as they develop significant stenoses prior to occlusion, but this is not always the case. However, duplex surveillance will allow for detection of patients progressing to failure and capture them prior to complete occlusion. Furthermore, the restoration of secondary patency (after occlusion of a previously stented SFA) is associated with a lower rate of technical success, and patients are at an increased risk for distal embolization when mechanical thrombectomy techniques are utilized [18, 19]. Conversely, endovascular treatment of patients with in-stent stenosis or new stenotic lesions proximal or distal to a previously treated lesion is relatively low-risk with a high rate of technical success. By combining PSV and velocity ratio data, criteria that are very specific and predictive for both 50% and 80% in-stent stenoses within the superficial femoral artery have been developed. Additionally, the set point which discriminates between these degrees of stenosis is very distinct. Thus, by applying these criteria at appropriate follow-up intervals, in-stent restenosis should be recognized prior to occlusion for the majority of patients.
Unfortunately, the natural history of in-stent restenosis lesions are not clearly defined in either the aortoiliac or femoropopliteal segments. Although it seems appropriate to offer patients secondary or tertiary endovascular procedures to avoid recurrent lifestyle-limiting claudication or severe rest pain, there are other scenarios which are less clear. For patients with CLI and tissue loss, who have had a successful intervention and appropriately healed a wound, is it necessary to reintervene to treat a >80% in-stent stenosis? The answer is unclear. As has been described in the surgical literature [20], additional studies will have to be performed to determine patient and pre-intervention lesion characteristics along with procedural characteristics that may help the efficacy of duplex surveillance in predicting failure of endovascular therapies in the lower extremities. Additionally, the duplex characteristics of these stented lesions will need to be studied further to determine if there are factors predictive of failure which can be evaluated on surveillance imaging.
Outcome Measures
As with other arterial beds which are followed using duplex surveillance, the utility of protocols to follow lower extremity endovascular interventions is dependent on standardized definitions and criteria. Unlike surgical bypass, whereby the diseased vessel is excluded from the circulation, with endovascular interventions, the diseased vessel remains the primary channel through which blood flows. Therefore, the definition of initial technical success of endovascular interventions varies from conventional surgical technical success and is reported as a residual luminal stenosis less than 30% compared to normal artery proximally by angiography or other imaging [21]. Furthermore, anatomic failure is reported as a restenosis of 50% or greater compared to normal diameter.
In addition to initial technical success, patency over time is also standardized in its definition. Primary patency is defined as uninterrupted patency of the treated vessel without any endovascular to open procedures to maintain or restore patency. Assisted primary patency is uninterrupted patency of the treated vessel with a secondary intervention (e.g., secondary angioplasty). Endovascular interventions performed proximally and distally to the initially treated lesion are included in these secondary interventions. Secondary patency is defined as patency of a vessel after an intervention was undertaken to restore patency following occlusion of a vessel. As alluded to previously, assisted primary patency and secondary patency rates are higher than primary patency rates following endovascular interventions [6–15], and one primary aim of surveillance is to optimize these higher rates with early recognition of failing interventions.
Technical Considerations of Surveillance Following Endovascular Interventions
When compared to surgical bypass, endovascular interventions differ in numerous ways which are of great importance when considering a surveillance protocol. Additionally, within the realm of lower extremity endovascular interventions, there are a plethora of different techniques and technologies which are used to restore normal arterial flow, all of which may lead to subtle differences that need to be taken into account in duplex surveillance.
Unlike surgical bypass, arterial flow is intentionally maintained through the normal anatomic course with endovascular interventions. As such, there are no proximal and distal anastomoses which need to specifically be surveyed for the development of intimal hyperplasia. However, just with surgical bypass, an important component of surveillance following endovascular interventions is assessment of the inflow and outflow vessels along with the proximal and distal segments of the treated artery itself. This may prove somewhat difficult when compared to surgical bypass as, often, only the most diseased portion of a vessel is selected to undergo intervention while the vessel proximally and distally may demonstrate some degree of disease, albeit not deemed hemodynamically significant at the time of the initial intervention and therefore left untreated. Additionally, endovascular interventions typically involve controlled trauma to the target lesion, whether via angioplasty or some other mechanism. With angioplasty specifically, the plaque is split and the media is stretched in an effort to increase the lumen size of the vessel. Along with this, there is a risk of embolization and creation of intimal flaps. Furthermore, these plaque fractures and dissection planes may extend proximally and distally to the lesion and create an environment susceptible to intimal hyperplasia.
Given the common anatomic presentation of peripheral arterial disease and its subsequent treatment using endovascular techniques, two specific factors regarding surveillance must be considered. First, as the proximal vessel may not be disease-free, velocities through these segments may be elevated. As such, in addition to evaluating peak systolic velocities (PSV), evaluating the ratios of PSVs at the site of intervention versus the PSVs proximally along with evaluation of the waveforms is of great importance. Second, the entire vessel must be surveyed as there is potential for progression of lesions proximally and distally and not just at the site of interventions.
Although the mainstay of endovascular treatment remains angioplasty with or without stenting, endovascular techniques for the treatment of lower extremity arterial occlusive disease are continuing to evolve with newer technologies. In particular, there are a variety of different atherectomy devices, which utilize rotating blades, laser-generated heat, or ultrasonographic energy to mechanically debulk plaque and restore arterial flow through the true lumen of the vessel. Additionally, conventional angioplasty may also maintain flow through the true lumen. However, often the technique of subintimal angioplasty is used whereby a new lumen is created and plaque is pushed away from this channel. An understanding of these differences in techniques is useful for post-intervention surveillance for both the surgeon/interventionalist and the vascular technologist, as specific anatomic factors related to the procedure may lead to higher rate of restenosis (e.g., reentry point of a subintimal angioplasty) and, subsequently, specific anatomic sites may warrant careful evaluation.
Surveillance Protocols
Our duplex surveillance protocol consists of the following: Patients are seen in follow-up at 1, 3, and 6 months after their procedure. After this initial 6-month period, patients are then evaluated at 6-month intervals indefinitely. Follow-up consists of an office visit with the treating physician and noninvasive studies including ankle-brachial indices, pulse volume recordings, and complete arterial duplex ultrasound examinations of the treated limb. If a patient undergoes a reintervention, then the protocol is reset from the time of the most recent intervention. For asymptomatic patients who are found to have mild stenoses either in the immediate post-procedure time or at some point during their follow-up, surveillance is transitioned to 3-month intervals. If these patients progress on to high-grade stenoses, as defined by our data, or become symptomatic, they will be offered reintervention. For patients who remain with stable mild asymptomatic lesions after 1 year, they are then transitioned to 6-month follow-up intervals. The importance of follow-up and the rationale behind surveillance is explained in detail to patients prior to their initial intervention and is reiterated at each visit. Certainly, noncompliance may be an issue, particularly for asymptomatic patients.
A standardized scanning protocol is used for patients who have undergone endovascular interventions. All duplex ultrasonography is performed by registered vascular technologists at laboratories approved by the Intersocietal Commission on Accreditation of Vascular Laboratories using either a LOGIQ 9 (General Electric Healthcare, Piscataway, NJ) or a LOGIQ e (General Electric Healthcare, Piscataway, NJ) system. A 7-MHz linear probe is most commonly used at an angle of insonation of 60°, or when not possible, angle correction is used. For evaluation of deeper structures in the abdomen, a low-frequency (2.5–4 MHz) or curved array transducer may be used. The entire treated vessel is examined along with one complete vessel above and one complete vessel below. The PSV is calculated at standardized points within the vessel examined in addition to areas of suspected stenosis. Furthermore, the PSV ratio is calculated at areas of stenosis from the PSV within the stenosis to the PSV within a minimum of at least 3 cm proximal to the stent in the native artery. Additionally, gray-scale imaging and visualization of the vessel and any indwelling stents is reviewed along with evaluation of spectral waveforms.
For aortoiliac interventions, color-flow Doppler imaging is obtained from the distal aorta along the iliac arteries and through the common femoral arteries, the profunda femoris arteries, and the superficial femoral arteries. Spectral velocity waveforms are measured, and waveforms and PSVs within treated segments are compared to velocities proximally in the native vessel. For femoropopliteal interventions, the common femoral artery, the femoral bifurcation, and the length of the superficial femoral artery, as well as the popliteal artery and proximal tibial vessels are imaged. As with iliac interventions, spectral waveforms and PSVs within treated femoropopliteal arterial segments are compared to those in the segments proximally.
Surveillance Criteria of Lower Extremity Endovascular Interventions
Previously, duplex and noninvasive criteria for the diagnosis of in-stent stenosis had been generalized from data concerning the detection of de novo lesions in previously untreated femoropopliteal vessels or in the detection of vein bypass graft stenosis. However, it has been shown that stent placement within an arterial segment results in a change in vessel compliance which may alter velocities as measured by duplex [22]. It has also been demonstrated that stent placement in the carotid circulation alters the velocity thresholds for the detection of significant recurrent internal carotid artery stenosis [23]. We have described criteria for the determination of in-stent stenosis after angioplasty and stenting of the superficial femoral artery and used these criteria for the follow-up of patients who have undergone endovascular lower extremity interventions [17]. At present time, there are no specific criteria for the determination of in-stent restenosis after iliac interventions, and, by convention, data from studies of untreated vessels and native stenoses have been applied.
To determine criteria of in-stent stenosis after angioplasty and stenting of the superficial femoral arteries, we reviewed all endovascular interventions for femoropopliteal occlusive disease between May 2003 and May 2008, during which time 330 limbs underwent femoropopliteal angioplasty and stenting. Data pairs of duplex and angiographically measured stenoses within 30 days of each other were analyzed. Angiograms were obtained in anteroposterior and oblique views at the time of initial and secondary or tertiary imaging. The view demonstrating the greatest degree of stenosis was used to determine the percentage of in-stent stenosis.
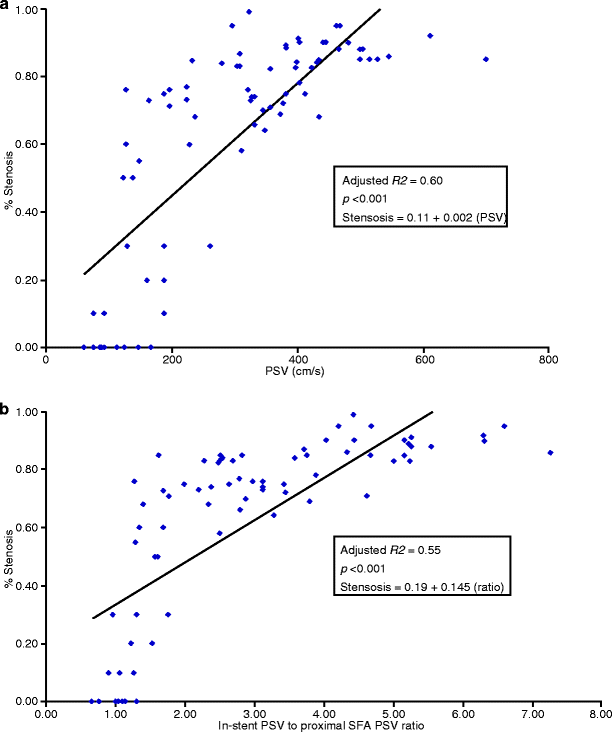
Linear regression analyses were performed, and receiver operator characteristic (ROC) curves were used to compare angiographic stenosis with PSV and stented SFA velocity/proximal SFA velocity ratio to determine optimal criteria equating to ≥50% and ≥80% stenosis. A linear regression model of PSV versus degree of angiographic stenosis showed a strong adjusted correlation coefficient (R 2 = 0.60, p < 0.001). Additionally, there was a strong adjusted correlation coefficient (R 2 = 0.55, p < 0.001) for velocity ratio versus degree of angiographic stenosis, but only a moderate adjusted correlation coefficient (R 2 = 0.31, p = 0.02) for decrease in ABI versus degree of angiographic stenosis (Fig. 26.2).