Increasing sheath size
Cannulation of artery other than common femoral artery
Calcified artery
Increased body mass index
Concurrent anticoagulation
Combined arterial and venous puncture
Failure to provide appropriate postoperative compression
The reported incidence of IPA ranges widely from 0.05% up to 9%. This wide incidence range is the result of variations in protocols used to assess IPAs. In a prospective study of over 500 patients with routine evaluations with duplex, an incidence of 7.7% was reported [3]. This figure is substantially larger than that seen in clinical practice, since only symptomatic patients are typically assessed. According to the Society of Cardiovascular and Interventional Radiology, an acceptable rate of IPA and/or arteriovenous fistula should be ≤0.2% [4].
Diagnosis
Clinical suspicion for IPA should follow any percutaneous intervention resulting in a swollen groin or soft tissue hematoma. Hard signs of persistent bleeding include pulsatile bleeding at the access site or expanding hematoma. The presence of a femoral bruit or thrill may also indicate an IPA; however, their absence does not exclude it. Most commonly, IPA is associated with a painful access site with associated hematoma and ecchymosis of varying size. Unfortunately, physical examination alone is notoriously inaccurate in identifying IPA. Angiography was historically the gold standard for diagnosis of IPA, until Mitchell et al. reported successful diagnosis by color duplex ultrasound in 1987 [5]. Since that time, arterial duplex evaluation has emerged as the gold standard and initial modality for diagnosing an IPA, with nearly 100% diagnostic accuracy. We typically use a 5- to 7-mHz probe in longitudinal orientation with vessel sampling and velocity measurements of the femoral artery and its branches. Changing orientation to the transverse plane allows diagnostic confirmation, sac size measurement, and evaluation for presence of thrombus within the pseudoaneurysm (Fig. 27.1). Duplex evaluation should include views of the inflow, distal external iliac, common femoral, deep femoral, and proximal superficial femoral arteries. Visualization of the common femoral vein is particularly important to exclude the presence of an associated arteriovenous fistula. IPA anatomic features to be reported should include maximum sac size, diameter of sac with flow, sac shape, neck diameter, and length. The sac size of the pseudoaneurysm should be measured in maximum squared centimeters, which is the most important parameter for determining treatment options. However, the length and width of the neck are also important to record. Larger neck widths often directly correlate with larger arterial defects that are generally more refractory to treatment with minimally invasive techniques. Multilobed IPA(s) likewise may be more difficult to treat. In our previous series, up to 20% of IPA(s) were multilobed. Typical characteristics noted on duplex imaging include a swirling of color flow within a hematoma outside of the underlying artery, color flow signal in a tract leading to a sac, and classic to-and-fro color flow in the pseudoaneurysm sac (Fig. 27.2).
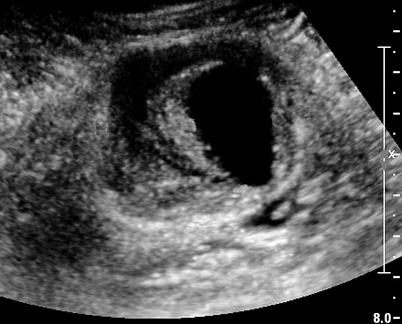
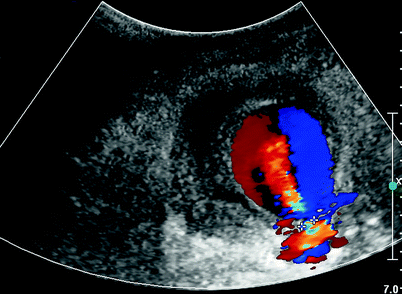

Fig. 27.1
B-mode image of preinjection partially thrombosed pseudoaneurysm

Fig. 27.2
Duplex imaging with to and fro flow. Pseudoaneurysm neck marked
Treatment Options
Observation
Several small studies have reported successful closure in more than 50% of pseudoaneurysms by observation alone; however, these studies were not able to identify variables that could accurately predict which IPA(s) could be safely observed. In a prospective study evaluating the natural history of femoral vascular complications following coronary catheterization, all puncture sites were evaluated with physical examination prior to patient discharge. This series reported observation of seven femoral pseudoaneurysms with maximum sac diameter ≤3.5 cm with no complications and 100% thromboses at 4 weeks [2]. In a series of similar size, 9 of 16 IPA(s) thrombosed with observation. Larger IPA(s) and those associated with anticoagulation were associated with more frequent failures in the patients observed [6]. The largest series of conservative management of patients with femoral pseudoaneurysms is reported by Toursarkissian et al. [7]. Eighty-two patients with IPA(s) were followed at 2, 4, 8, and 12 weeks with duplex imaging of the femoral artery. A spontaneous thrombosis rate was observed in 89% of patients with no adverse events noted during observation. The mean time for spontaneous closure was 23 days, with mean 2.6 duplex examinations per patient performed [7]. Exclusion criteria for observation included IPA(s), >3 cm, concurrent anticoagulation, severe pain, or inability to comply with recommended follow-up examinations. Unfortunately, adoption of an observation policy for small IPA(s) (1–3 cm) does raise some concerns. These concerns included compliance with follow-up, patient fears of aneurysmal rupture (especially those with previous diagnosis of aneurysm), and finally costs to the health care system. These concerns have modified our management strategy in select patients with pseudoaneurysms in this size range.
Ultrasound-Guided Compression
Until the early 1990s, femoral cutdown with suture repair of the femoral defect was the standard treatment for those not managed conservatively. In 1991, Fellmeth et al. described a nonoperative technique for thromboses of IPAs and arteriovenous fistulas. With an overall success rate of 93%, this noninvasive alternative to surgical repair was welcomed [8]. Following this initial report, the use of ultrasound-guided compression (UGC) became the first-line treatment for those who were hemodynamically stable and without associated infection or skin necrosis. This technique includes a linear or curvilinear probe (5 or 7 MHz) to compress the IPA and arrest IPA blood flow. Real time, duplex, and color Doppler are used to identify the neck of the pseudoaneurysm. Manual compression is subsequently applied by the technologist to the neck of the aneurysm with the transducer, permitting flow through the native artery while preventing flow into the pseudoaneurysm sac. Continuous evaluation during compression is essential to arrest flow in the pseudoaneurysm sac and ensure flow in the native artery. Pressure is maintained for 10-min intervals, at the end of which pressure is slowly released and flow into the pseudoaneurysm reassessed. This is continued until thrombosis, operator fatigue, or patient discomfort occurs. Success with this treatment modality generally ranges from 60% to 90% [9–11]. Despite this acceptable success rate, compression times in excess of 1 h can be required and multiple compression sessions may be required to induce thrombosis of >10% of IPA(s). Factors associated with failed compression have been evaluated in previous publications. Ongoing anticoagulation has been shown in several series to significantly reduce successful compression, as reported by Coley et al. [10] and Eisenburg et al. [12]. They describe failure rates of 38% and 70%, respectively, in anticoagulated patients, whereas failure rates in the groups without concurrent anticoagulation were 5% and 26%. In addition, 75% of those in Coley’s series ultimately had their anticoagulation stopped and underwent repeat UGC with successful thrombosis. Also a series by Dean et al. reported a 73% success rate of UGC in 77 patients with uninterrupted anticoagulation, with seven patients requiring multiple sessions (12.5%) to obtain sustained thrombosis. Maximum aneurysm diameter appeared to be the best predictor of successful treatment with UGC [13]. With increasing pseudoaneurysm sac size, the technical success of ultrasound-guided compression decreases. Coley et al. achieved a 100% success rate in pseudoaneurysms <2 cm but only a 67% success with UGC in those 4–6 cm. Although it seems intuitive that a shorter tract length and larger neck diameter would have less success with compression, this has not been widely reported. Limited data is available on neck length or diameter on success of ultrasound-guided compression. A small series reported a short tract length (<5 mm) had unfavorable compression outcomes; however, this study was limited by a small sample size of only 12 patients [14].
Complications following UGC include arterial or venous thrombosis. Also, several cases of pseudoaneurysm rupture have also been reported. Successful thrombosis occurs at an acceptable rate, but there are several limitations to this technique. These include the requirement at some facilities for a physician to be present during the entire procedure, potential long procedures times, local patient discomfort that often requires conscious sedation, and newer techniques with higher success rates now being available.
Ultrasound-Guided Thrombin Injection
In 1986, Cope and Zeit described a technique of ultrasound-guided percutaneous injection of bovine thrombin into a pseudoaneurysm sac with successful thrombosis [15]. This technique was actually described prior to the adoption of ultrasound-guided compression. Although an appealing method for handling IPA, this technique took over a decade to widely replace ultrasound-guided compression. During that time interval, several authorities have compared their experience of ultrasound-guided compression to that of ultrasound-guided thrombin injection (UGTI) [16–22] (Table 27.2). UGTI has greater technical success in all series included in this review. All series however are retrospective series except a small prospective randomized trial by Lonn et al. with 15 patients in each group [22]. In addition to great immediate technical success, the subsequent recurrence rate is also low [23–36].
Table 27.2
Ultrasound-guided compression (UGC) versus ultrasound-guided thrombin injection (UGTI)
Reference | N (UGC)/(UGTI) | Success (%) (UGC)/(UGTI) |
|---|---|---|
Weinmann et al. [16] | 30/33 | 87/100 |
Gorge et al. [17]a | 36/30 | 17/93 |
Taylor et al. [18] | 40/29 | 63/93 |
Stone et al. [19] | 47/27 | 57/96 |
Paulson et al. [20] | 281/26 | 74/96 |
Khoury et al. [21] | 189/131 | 75/96 |
Lonn et al. [22]b | 15/15 | 40/100 |
UGTI can be performed at bedside or in the vascular laboratory. Some clinicians use local anesthetic; however, with experience single punctures can be achieved and conscious sedation has never been required in our experience. A 1-ml syringe and spinal needle (20–22 gauge) is used to administer the bovine thrombin (100–1,000 units/ml). Using B-mode imaging, the needle tip is visualized and directed into the IPA sac (Fig. 27.3). The interventionalist should ensure the needle tip is just inside the sac and as far from the IPA neck as technically possible. This minimizes the chance of forcing thrombus or thrombin into the neck and the native circulation. Injection is performed into the sac under duplex visualization at 0.1-ml increments until successful obliteration of flow into the aneurysm sac is achieved (Fig. 27.4). Thrombin is an active form of factor II (prothrombin). It transforms inactive fibrinogen to fibrin, its active form. Fibrin subsequently contributes to thrombus formation. As a result of limited blood flow in the pseudoaneurysm sac, thrombin can propagate thrombus that would often be cleared in sites of normal blood flow. After successful thrombosis, the native circulation is assessed for changes compared to the preprocedural status. Following successful thrombosis, bed rest is recommended for a period of 4–8 h.
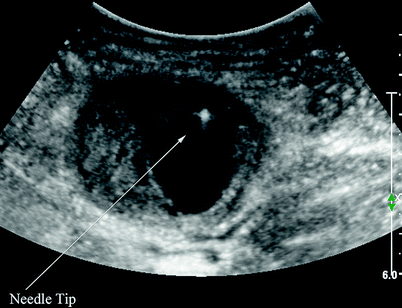
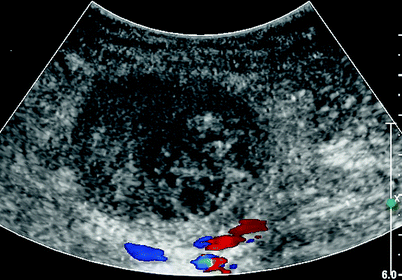

Fig. 27.3
Needle tip located away from pseudoaneurysm neck

Fig. 27.4
Duplex imaging successful of thrombosis pseudoaneurysm. Absent color flow visualized
Complications following thrombin injection are reported infrequently. The most feared complications are arterial thrombosis and distal embolization. Distal embolization has occurred in less than 1% of the reviewed literature, with some authors noting improved circulation spontaneously. Others have been successfully treated by intra-arterial thrombolytic therapy or surgical thrombectomy. Limited evidence suggests that distal embolization is associated with short and wide pseudoaneurysm necks. Also, most cases reported in the literature have occurred in aneurysms with maximum sac diameter <3 cm. Other complications seen only in case reports include allergic reactions to bovine thrombin with severity ranging from generalized urticaria to anaphylaxis. Infection after injection has also been reported occasionally, and one case of rupture was identified in a comprehensive literature review.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


