Type 1 HP (e.g., farmer’s lung)
Type 2 HP (e.g., bird fancier-s lung)
Exposure
Usually massive and intermittent
Usually chronic insidious
Usually microorganisms (fungi and actinomycetes)
Usually lowdose of avian antigens
Clinical behaviour
Primarily acute/subacute : higher frequency of fever and recurrent episodes. Usual phlegm
Recurrent BFL: cough and mild exertional dyspnea, low-grade fever
More recurrent systemic symptoms (chills, body aches)
Insidious BFL: progressive dyspnea; clubbing
Lung function tests
Mild restrictive abnormalities that resolve
Restrictive pattern
Airflow obstruction (usually mild) seen in chronic disease
Hypoxemia at rest or exercise common
Lung imaging studies
Chest X-ray: frequently normal
Chest X-ray: frequently abnormal
HRCT: ground glass opacities, predominating in the lower lobes, fine centrolobular nodules, hyperluscent areas
HRCT: irregular reticular opacities, traction bronchiectasis and honeycombing superimposed to subacute changes (e.g. ground-glass opacities, nodules, hyperluscent areas)
Most frequent long-term sequelae: mild emphysema often sparing the upper parts of the lung
BAL and precipitins
Non-specific for differentiating both types
Non-specific for differentiating both types
Lung biopsy
Small, poorly-formed noncaseating granulomas located near bronchioles
Ill-formed granulomas (may be difficult to identify)
Peripheral airways: proliferative bronchiolitis obliterans, characterized by fibroblast proliferation and an organizing intraluminal exudate that occludes bronchioles from within
Fibrotic pattern: NSIP-pattern or UIP-like pattern
Peripheral airways: constrictive bronchiolitis
Outcome
Usually resolves
Poor, often progress to fibrosis
Chronic exposure may lead to chronic bronchitis or emphysema
Possible acute exacerbation of chronic form without further exposure
Diagnostic Methods
There are no specific tests or biomarkers to date that allow a consistent diagnosis of HP. Therefore, it is, by necessity, based on the conjunction of clinical, radiological, functional, immunological and histopathological diagnostic indicators among which none is actually specific to the disease. Of these, histopathological signs are the most reliable but open-lung biopsy is more and more rarely performed. HRCT scans and data from bronchoalveolar lavage (BAL) are considered to be the most useful non-invasive tools. The first often show pathognomonic features; the second is highly sensitive. Finally, in absence of standardization in the inhalation protocol and in the criteria defining a positive response, specific provocation tests are not recommended.
Histopathology
Most cases of HP, whether acute or subacute, include the following histologic features in variable proportions [21]:
1.
cellular bronchiolitis, which is the presence of chronic inflammatory cells lining the small airways, sometimes with resultant epithelial ulceration;
2.
diffuse chronic interstitial inflammatory infiltrates, primarily consisting of lymphocytes and plasma cells but often including eosinophils, neutrophils and mast cells;
3.
poorly circumscribed interstitial non-necrotizing (non-caseating) granulomas consisting of lymphocytes, plasma cells and epithelioid histiocytes, with or without giant cells (Fig. 29.1). Both the interstitial mononuclear and the granulomatous inflammation tend to form around bronchioles and obliterative bronchiolitis may occur (Fig. 29.2). Scattered areas of organizing pneumonia with intraluminal bronchiolar polyps also are common;
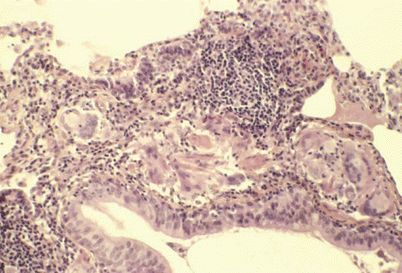
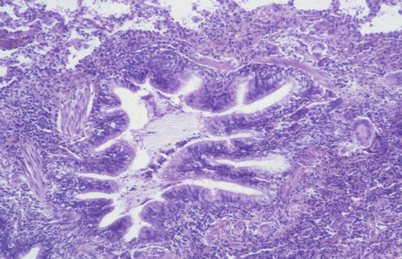

Fig. 29.1
Peribronchiolar poorly formed granuloma with giant cells and numerous lymphocytes. Absence of follicular organization. Type 1 farmer’s lung disease

Fig. 29.2
Interstitial mononuclear and granulomatous infiltration around the bronchiole giving a constrictive bronchiolitis feature. Hypersensitivity pneumonitis due to moulds on a ceiling
4.
individual giant cells in the alveoli or interstitium. These cells may contain inclusions of endogenous metabolic products, such as cholesterol clefts, Schaumann bodies and lucent birefringent oxalate crystals.
The bronchiolitis may include variable degrees of peribronchiolar fibrosis and hyperplasia of the bronchiolar epithelium, a characteristic but non-specific finding [22].
In some patients with insidious onset disease, emphysema may be a prominent component. Non-specific interstitial pneumonitis (NSIP), usual interstitial pneumonia (UIP) and bronchiolitis obliterans organizing pneumonia might be the sole histological expressions of the disease [20]. In patients in whom the pattern of fibrosis is consistent with UIP or NSIP, the presence of giant cells, granulomas, areas of interstitial granulomatous inflammation, or peri-bronchiolar fibrosis should suggest the diagnosis of HP.
Imaging Features
Chest radiography is used to determine the cause of illness and rule out the others. Up to 20 % of individuals with active HP have normal chest X-rays [23].
HRCT reflects histopathologic findings with precision by showing direct (mainly centrilobular opacities and ground-glass opacities) and indirect (mainly hyperluscent area and air-trapping) images.
Ground-glass opacities are usually bilateral and symmetric, but sometimes patchy; they generally predominate in the middle part of the lungs. These opacities usually represent chronic interstitial inflammation but occasionally may be caused by fibrosis or organizing pneumonia. Another characteristic direct feature is the numerous small round centrilobular opacities, usually less than 5 mm in diameter (Fig. 29.3). These centrilobular ground-glass opacities sometimes have relatively well-defined borders and are referred to as nodules. These abnormalities represent cellular bronchiolitis, peribronchiolar interstitial inflammation, or, less frequently, focal organizing pneumonia. The third major finding is hypoattenuation and hypovascularity of scattered secondary lobules giving sometimes a mosaic aspect (Fig. 29.4). Hypoattenuating regions that persist on expiratory HRCT are indicative of air-trapping (Fig. 29.5), which is caused by bronchiolar obstruction, often favored by the presence of mucus. The combination of patchy ground-glass opacities, normal regions and air-trapping on HRCT is sometimes referred to as the headcheese sign [21]. Bronchiolar wall thickening may also occur. Lung cysts have been found occasionally and are probably caused by obstruction of bronchioles (Fig. 29.5). Focal consolidation, which often represents organizing pneumonia, is rarely present. Mediastinal lymph-node enlargement does not rule out the diagnosis of HP [17]. Pulmonary arteries are occasionally enlarged, presumably reflecting pulmonary arterial hypertension [24]. In type-1 patients, especially farmer’s lung patients, centrilobular emphysema may develop [17]. When fibrosis is present, HRCT demonstrates reticulation, mainly in the middle portion of the lungs or evenly throughout the lungs but with sparing of the extreme bases [21, 25]. Honeycombing may occur, resembling that in patients with idiopathic pulmonary fibrosis (IPF) [26]. When fibrotic HP is compared with IPF or NSIP, the CT-features favoring a diagnosis of HP are lobular air-trapping, centrilobular ground-glass opacities and absence of lower-lobe predominance [21, 26].
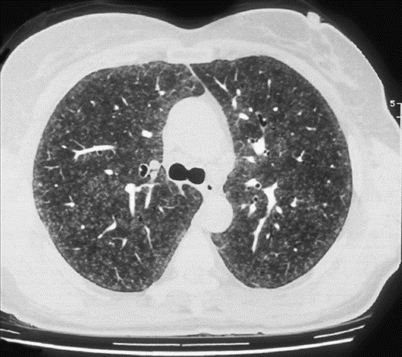
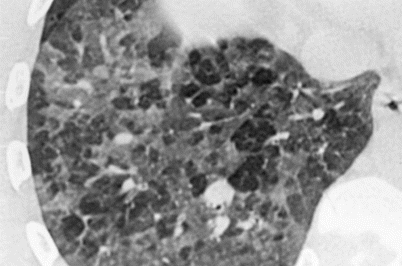
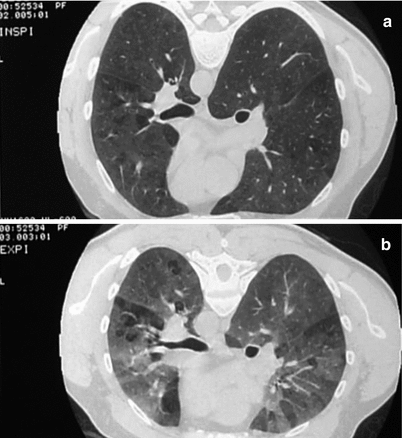

Fig. 29.3
Diffuse ill-defined centrilobular small nodules. Farmer’s lung disease

Fig. 29.4
Ground-glass opacities and hypoattenuation of scattered secondary lobules giving a mosaic pattern. Hypersensitivity pneumonitis due to molds in wood sewage

Fig. 29.5
Bilateral ground-glass opacities, associated with hyperlucent segmentar areas (a). Expiratory slices show an accentuation of density of ground-glass without loss of volume of hyperlucent aeras signing air trapping due to bronchial obstruction (b)
Bronchoalveolar Lavage
BAL plays a major role in the investigation of patients suspected of having HP in that a normal number of lymphocytes rules out all but residual disease [27]. BAL is considered normal when the percentage of lymphocytes is less 30 % in non-smokers and 20 % in smokers. Nevertheless, a significant increase in neutrophils can be observed when BAL is carried out shortly after exposure [28]. In this situation, the BAL sample can consist mainly of macrophages and neutrophils, with less than 30 % of lymphocytes. Soon afterwards, lymphocytes predominate. Lymphocytosis is also seen in BAL fluid from patients with a history of HP but with no recent activity, making it a very instructive tool for retrospective diagnosis. BAL lymphocytosis is, however, poorly specific as it may be seen in many other lung diseases [29]. A classic finding is a ratio of CD4 to CD8 lymphocyte subsets that is less than 1 (the normal ratio is 1.8). CD4/CD8 ratio is probably affected by the clinical form of HP, exposure to tobacco smoke, the type and dose of inhaled antigen and the time elapsed since antigen exposure. A recent multicenter study in France retrospectively assessed the diagnostic value of BAL in 139 HP cases, mainly farmer’s lung: only 34 % of the subjects showed a CD4/CD8 ratio less than 1 [30]. Hence, the usefulness of the CD4/CD8 ratio to diagnose HP is highly questionable.
Specific Serum Antibodies
The presence of circulating antibodies against the putative offending antigen(s) is useful for diagnosis. Indeed, the results of the HP study demonstrated that positive serum antibodies are a significant predictor of HP (odds ratio: 5.3; 95 % IC: 2.7–10.4) after taking antigen exposure into account [4]. The selection of antigens to be tested often needs to be determined locally according to the prevalent antigens. In Eastern France, by using a panel of antigens shown to be responsible for farmer’s lung rather than a classic standardized panel, serological tests showed high figures for both negative predictive values and specificity (from 89 to 95 % according to prevalence of the disease). In that study however, sensitivity was low with 11 out of the 31 tested HP cases negative. Additional investigations by microbiological samplings in the environment of these negative subjects allowed to identify antigens not present in the initial panel. Eight of the 11 false-negative HP cases became positive after testing these unusual antigens. These results highlight the importance of (1) the use of antigens proven to be representative of exposure, (2) the knowledge of unusual antigenic exposure and (3) sampling the patient’s environment.
Several methods of determining precipitins or total IgG antibodies have been described. Enzyme-linked immunosorbent assay (ELISA) is largely used because this technique is standardized. The consistency of 4 serological techniques (electrosyneresis, Ouchterlony double-diffusion, ELISA and Western-blot) was recently evaluated in France. Electrosyneresis on cellulose acetate was the most relevant diagnostic tool to discriminate farmer’s lung from healthy exposed farmers (sensitivity 87 %, specificity 100 %) [31].
All of these considerations concern tests against organic antigens: microorganisms, moulds or animal proteins. In the case of HP due to chemical substances, isocyanates for example, serological tests have not been demonstrated to be useful or even interpretable.
Pulmonary Function Tests
Pulmonary function tests (PFTs) have no discriminative properties in differentiating HP from other diffuse infiltrative lung diseases [4]. At the time of diagnosis, PFTs can be normal, or classically show a restrictive pattern (especially in type 2 HP) or sometimes reveal an obstructive pattern. In chronic disease, the pattern can be restrictive, but at least in farmer’s lung, and probably in other HP due to microorganisms, the most frequent profile is an obstructive defect resulting from chronic airway involvement or emphysema [32]. Decreased carbon monoxide diffusion capacity (DLCO) is generally considered to be consistently present in HP [33, 34]. But if we strictly define abnormal values as a DLCO <80 % predicted, some HP cases have normal results [3]. Importantly, the improvement of DLCO is very low, for up to 2 years in the large follow up study by Kokkarinen et al. [34]. This slow recovery of DLCO is in agreement with the slow recovery of both histological changes and lymphocytic infiltration in HP. Hypoxemia is common, especially with exercise. Hypoxemia (at rest or at exercise) and lung volume defect recover quickly, within a few months after an acute episode [34, 35]. These abnormalities are nevertheless non-specific since similar changes are found in most interstitial lung diseases (ILDs).
Diagnostic Criteria
HP represents a diagnostic challenge due to the absence of any one feature that distinguishes it from other ILDs. The diagnosis of HP relies on a high degree of clinical suspicion, the recognition of past history of antigen exposure and conjunction of clinical, radiologic, laboratory and sometimes pathologic findings [9]. Several diagnostic criteria recommendations have been published but none has been validated, so their diagnostic accuracy is unknown [36]. The HP Study [4] is, to our knowledge, the only one to give validated diagnostic criteria. But, it evaluated non-invasive clinical criteria only. In addition, it included a large proportion of the chronic form of HP induced by continuous avian exposure, which might make it difficult to extrapolate and apply the results to HP in general.
The HP Study
The objective of this prospective multicenter cohort study was to develop a clinical prediction rule for the diagnosis of active HP. Such a rule aims at helping clinicians to reach a more accurate estimate of probability of HP and to decide whether further investigation is needed to rule it either in or out [36]. Consecutive adult patients presenting with a pulmonary syndrome for which active HP was considered whatever the possible diagnosis were included in the study. The investigators had to classify each patient as HP or non-HP. The final diagnosis was based on BAL findings, HRCT and, when necessary, other procedures including surgical lung biopsy.
Multivariate analysis identified 6 significant independent predictors of HP (Table 29.2). The clinical prediction model produces an equation that expresses the probability of HP as a function of the statistically significant variables. From this equation, a table of probability for combinations of predictors was constructed (Table 29.3). For instance, farmers presenting with recurrent episodes of respiratory symptoms, inspiratory crackles and positive testing for the corresponding precipitating antibodies, the probability of HP would be 81 % (Table 29.3). A patient with progressive dyspnoea and inspiratory crackles as the only criteria in favor of HP would have a probability of less than 1 % [36]. Further investigations such as HRCT and/or BAL would be indicated in the first case; diagnosis would be excluded in the second.
Table 29.2
Significant predictors of hypersensitivity pneumonitis
Variables | Odds ratio | Confidence interval (95 %) |
|---|---|---|
Exposure to a known offending antigen | 38.8 | 11.6–129.6 |
Positive precipitating antibodies | 5.3 | 2.7–10.4 |
Recurrent episodes of symptoms | 3.3 | 1.5–7.5 |
Inspiratory crackles | 4.5 | 1.8–11.7 |
Symptoms 4–8 h after exposure | 7.2 | 1.8–28.6 |
Weight loss | 2.0 | 1.0–3.9 |
Table 29.3
Probability (%) of having hypersensitivity pneumonitis
Crackles | |||||||
|---|---|---|---|---|---|---|---|
+ | − | ||||||
Serum precipitins (%) | Serum precipitins (%) | ||||||
Exposure to a known offending antigen | Recurrent episodes of symptoms | Symptoms 4–8 h after exposure | Weight loss | + | − | + | − |
+ | + | + | + | 98 | 92 | 93 | 72 |
+ | + | + | − | 97 | 85 | 87 | 56 |
+ | + | − | + | 90 | 62 | 66 | 27 |
+ | + | − | − | 81 | 45 | 49 | 15 |
+ | − | + | + | 95 | 78 | 81 | 44 |
+ | − | + | − | 90 | 64 | 68 | 28 |
+ | − | − | + | 73 | 33 | 37 | 10 |
+ | − | − | − | 57 | 20 | 22 | 5 |
− | + | + | + | 62 | 23 | 26 | 6 |
− | + | + | − | 45 | 13 | 15 | 3 |
− | + | − | + | 18 | 4 | 5 | 1 |
− | + | − | − | 10 | 2 | 2 | 0 |
− | − | + | + | 33 | 8 | 10 | 2 |
− | − | + | − | 20 | 4 | 5 | 1 |
− | − | − | + | 6 | 1 | 1 | 0 |
− | − | − | − | 3 | 1 | 1 | 0 |
The Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERM’O’P) French Propositions for Diagnosis Management
These propositions cannot be considered as actual diagnostic criteria in that they have not been validated. They represent a tool for standardized diagnostic management to be used by specialists of interstitial lung diseases (Table 29.4).
Table 29.4
Suggested working diagnostic criteria of hypersensitivity pneumonitis
1. Exposure to offending antigens: revealed by historya and/or microbiological investigations of the environment and/or presence of serum precipitins against antigen(s) from a standardized panel of antigen(s), or against antigen(s) present in the own environment of the individual |
2. Symptoms compatible with HP and basal crepitant ralesb |
3. BAL fluid lymphocytosisc |
4. Findings compatible with HP on HRCT |
5. Decreased DLCO and/or arterial hypoxemia (or decreased blood saturation) at rest or during exercise |
Their use requires:
access to an extensive list of causes of HP such as that used by the GERM’O’P, summarized in Table 29.1. This list of causes should be used in questionnaire form and should include some details on the circumstances of exposure as well as on the antigens responsible for the disease;
the presence of a reference laboratory able to do environmental samplings, to perform immunodiagnosis techniques including the production of antigens and the use of appropriate immunological methods;
HRCT with thin slices, procubitus and expiratory manoeuvres in order to detect indirect signs of bronchiolitis which may be the only abnormality in HP.
Rare, Unusual and New Causes of Hypersensitivity Pneumonitis
The Track of Offending Antigen(s)
Tracking the culprit antigen(s) is the most important thing to do when a patient presents with a respiratory syndrome for which HP is considered among the possible diagnoses. When the antigenic source is identified, the probability that a patient is suffering from HP is multiplied by 18 as compared with a patient for whom no exposure is found [4]. The presence of serum precipitins (or IgG) directed against the antigen(s) multiplies the probability of HP by 5 again [4]. Furthermore, when HP is diagnosed, identifying the precise etiology facilitates the only efficient treatment – avoiding the antigenic source. It is therefore crucial to be able to refer to a comprehensive and easily understood list of rare or newly identified causes.
However, identification of a specific antigen should not be a sine qua non for diagnosis, because new knowledge about antigens is generated from identifying new sources of exposure [2]. Some authors speak of HP from “unidentified causes”. In our opinion, it would be more appropriate to say HP from “causes not yet identified”.
In addition to the unusual etiologies or etiological circumstances presented in Table 29.5, we present below a brief overview of recently discovered or poorly known causes or circumstances of HP.
Table 29.5
Rare causes or etiological circumstances in Hypersensitivity pneumonitis (HP)
Diseases | Sources | Antigens |
|---|---|---|
(a) Hypersensitivity pneumonitis due to microorganisms and molds | ||
HP in agriculture and in related activities (other than classical farmer’s lung due to thermophilic actinomycetres and micromycetes that develop in moldy hay or straw or cereals) | Air-conditioner in a tractor Handling wheat during harvest Fertilizer and contaminated vegetals Weevils in cereals Compost (horticulture, market gardening…) Grain silo Endive workers Potato and onion workers Potato riddling Moldy wood chips in orchid plants Moldy rockwool used in rose plants | Gram-negative bacteria Ervinia herbicola Rhizopus sp. Aspergillus flavus Streptomyces albus Sitophilus granarius Aspergillus sp. Saccharomyces cerevisiae Fusarium sp. Fusarium solani Pencillium sp. Thermophilic actinomycetes Aspergillus sp. Cryptostroma corticale Aspergillus niger |
Mushroom worker’s lung due to spores of exotic species in Japan and in Europe (HP can also be related to exposure to thermophilic actinomycetes or micromycetes that develop in compost) | Puffball Oyster Pholiota Shiitake Enoki Tricholoma Bunashimati Maitaki Strophariaceae | Lycoperdon Pleurotus eryngii Pleurotus pneumonalis Pleurotus cornucopiae Pleurotus ostreatus Pholiota nameko Lentinus edodes Penicillium citrinum Lyophillium aggragatum Tricholoma conglobatum Hypsizigus marmoreus Grifola frondosa Strophariaceae |
Cheese worker’s lung | Molds (and sometimes mites) on various types of cheese | Penicillium casei Pencillium roqueforti Penicillium camemberti Penicillium crysogenum Acarus siro |
Malt worker’s lung | Moldy malt, moldy barley | Aspergillus fumigatus Aspergillus clavatus |
Suberosis | Moldy cork (woodman or cork workers) | Penicillium frequentum Penicillium glabrum Chrysonilia sitophila Aspergillus fumigatus |
Sequoiosis | Moldy redwood dust | Aureobasidium sp. Graphium sp. Aarthrinium phaeospermum |
Peat moss worker’s lung | Packing of peat moss | Monocillium sp. Penicillium citreonigrum |
Bagassosis | Molds in sugar cane | Thermoactinomyces vulgaris Laceyella sacchari Likely others actinomycetes |
Tobacco worker’s lung | Molds in tobacco leaves (tobacco industry) | Aspergillus fumigatus |
Thatched roof disease (new guinea) | Molds in the roof | Saccharomonospora viridis |
Paprika worker’s (or splitter’s) lung | Paprika dust | Mucor stonolifer |
Dry sausage worker’s lung Salami brusher’s disease Chacineros lung | Sausage powdering or labelling. Salami brushing. Molds on cold cuts | Pencillium camenberti Penicillium nalgiovense Penicillium candidum
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|