Pattern of DI involvement
Typical timing to onset
Develops in
Typical drugs or compoundsa
Main complications
Angioedema and UAO
Immediate-to-late
min-h
ACEI, ARB
UAO, throat closure, locked airway, asphyxia
Anaphylaxis
Immediate
min
Drugs, biologics, RCM, latex
Bronchoconstriction, shock, pulmonary edema
Laryngospasm
Immediate
sec
Asphyxia, NPPE
Sudden catastrophic bronchospasm
Immediate
min
ß-blockers, NSAID, aspirin, muscle relaxants, abused drugs
Locked airway, ARF, hypoxia, brain death
Acute intraoperative DI respiratory problem
Intraoperative, immediate
sec-min
See specific table
Anaphylaxis, acute bronchospasm, death
Flash pulmonary edema
min
min
Adrenaline, abused drugs, chemo, RCM
Hypoxemia, ARDS
Noncardiac pulmonary edema
min-h up to (months with oral drugs)
min-h
Chemo, hydrochlorothiazide, salicylate (dose-related)
Hypoxemia, ARDS
ARDS-DAD
d-y
h-d
Amiodarone, bleomycin, blood, chemo agents, paraquat
Hypoxemic ARF
TKI, oxygen, radiation therapy
May evolve to pulmonary fibrosis
Alveolar hemorrhage
d-mo
h-d
All anticoagulants, platelets inhibitors, cocaine, PTU
ARDS, clotting in central airway
Dense acute interstitial lung disease
w-y
h-d
Methotrexate
Hypoxemic ARF/ARDS
Acute amiodarone pulmonary toxicity
d-y
h-d
Amiodarone
Hypoxemic ARF/ARDS. Late pulmonary fibrosis
Acute eosinophilic pneumonia
d-mo
h-d
Minocycline, cocaine, venlafaxine, tobacco/marijuana smoke
Hypoxemic ARF/ARDS
Acute radiation-induced lung injury
w
d-w
Radiation therapy to the chest
Hypoxemic ARF/ARDS
Catastrophic pulmonary hypertension
Intraoperative, immediate
min
Protamine
Acute RVF, hypoxemia
Acute foreign body embolism
h-d
h-d
Lipids, silicone, hyaluronate, acrylate cement
Acute RVF/ARF/ARDS
Opportunistic infection
w-y
d
Corticosteroids, immunosuppressants, anti-TNF
ARF/ARDS (undistinguishable from DIRD)
Massive pleural/pericardial effusion/bleeding
w-y
h-d
Dantrolene, lupus-inducing drugs, all anticoagulants
Compression/tamponade
Acute methemoglobinemia
h-d
min-h
Benzocaine, dapsone, nitrites, oxidizing/occupational agents
Tissue hypoxia, pulmonary edema, brain damage
Ventilatory arrest
Immediate
sec
Opiates, colimycin
Tissue hypoxia
Neuromuscular failure
h-d
min-h
Aminosides, curares, dantrolene, narcotics
Hypoxemic ARF
Acute left ventricular failure
h-d
h-d
Doxurubicin, fluorouracil
Pulmonary edema
Multiple organ dysfunction syndrome
w-mo
d
Drugs that induce DRESS
Multi-organ dysfunction/failure
Drug-induced adverse reactions occur unexpectedly in a few individuals who are predisposed often without a clear reason for it, who receive the drug at normal dosage. There are usually little or no annunciating symptoms. Strictly speaking, drug overdosing is outside the province of drug-induced adverse effects, although overdoses of drugs can cause pulmonary edema, acute respiratory failure, the adult respiratory distress syndrome (ARDS), diffuse alveolar hemorrhage (DAH) and death. In addition, the limit between normal and above-normal dosage is blurred for such drugs as oral anticoagulants, thrombolytic agents, amiodarone, cyclophosphamide, bleomycin, nitrosoureas, salicylate and drugs of abuse, and for practical reasons respiratory injury associated with drug overdose will be covered here inasmuch as aging, impaired hepatic or renal function, drug interactions and/or variability in drug metabolism and clearance may alter drug disposition. Drug abuse and misuse (e.g. intravenous injection of crushed tablets intended for oral use) and chemical-induced respiratory injury are also covered here and in Pneumotox. Other complications from drugs include drug withdrawal-induced flare of the underlying condition, as shown by relapse of amiodarone pulmonary toxicity (APT) upon corticosteroids withdrawal, rebound respiratory failure following naloxone reversal of opioid intoxication [4], rebound pulmonary hypertension (PHT) following inadvertent cessation of pulmonary vasodilator therapy [5], or the recently-described ruxolitinib withdrawal syndrome [6].
Reports on drug-induced respiratory disease (DIRD) appeared as early as the late nineteenth century. The topic grew in the 1950s–1970s, and in 1973 about 120 drugs were known to injure the respiratory system [7]. This figure was propelled since the 1990s to 950 iatrogenic offenders at large, 15 main and 200 subpatterns. Classic offenders such as angiotensin-converting enzyme inhibitors (ACEI), amiodarone, anticoagulants, beta-blockers, bleomycin, cyclophosphamide, nitrosoureas and other chemo agents, blood transfusions, ergots, irradiation, methotrexate, minocycline, nitrofurantoin, nonsteroidal antiinflammatory drugs (NSAIDs) and salicylate still cause a persistent background of DIRD, although the current literature may not reflect it well as these adverse effects get rarely published nowadays. Drug-induced respiratory disease has garnered further interest as amiodarone, and novel biologicals and targeted agents such as tyrosine (TKI) or proteine kinase inhibitors (e.g. BCR-ABL), TNF-alpha antagonists, anti-CD20 (rituximab), inhibitors of the mTOR pathway, of platelet glycoprotein IIB/IIIA receptors, and direct antithrombin anticoagulants and reverse transcriptase inhibitors are now available to treat coronary artery disease, solid and hematologic malignancies, rheumatoid arthritis and other connective tissue disease (CTD), and systemic vasculitis. Targeted agents may cause on- and off-target adverse effects including but not limited to opportunistic infections [8, 9]. Exposure to these agents has been temporally related to the development of ILD, rapidly-progressive ILD, organizing pneumonia, eosinophilic pneumonia, sarcoidosis-like condition, diffuse alveolar hemorrhage (DAH), hypersensitivity, and anaphylaxis. Drugs, abused drugs, biologicals and silicone may also cause autoimmunity or autoimmune conditions [10, 11] with possible lung or serosal involvement including but not limited to lupus erythematosus, ANCA-related granulomatosis and polyangiitis (formerly known as Wegener’s), PR3- or MPO-antineutrophil cytoplamsic antibody (ANCA)-related vasculitis [12], eosinophilic vasculitis (Churg-Strauss’), Goodpasture-like pneumorenal syndrome, inflammatory myopathy and the anti-phospholipid syndrome. Drug-induced systemic conditions may manifest in the chest exactly as they do when they occur idiopathically in the form of ILD, DAH, eosinophilic pneumonia, lung nodules, lymphadenopathy, pleuropericardial effusion, systemic myositis or pulmonary thromboembolism. Biologicals used to treat rheumatoid arthritis also interfere with TNF signaling pathway causing opportunistic pulmonary or and/or systemic infections including reactivation of latent tuberculosis infection (LTBI) [13].
The possibility of exposure to illicit drugs should also be raised in patients presenting with acute pulmonary edema, diffuse alveolar hemorrhage, upper airway injury/burns, ILD, acute eosinophilic pneumonia, organizing pneumonia, pulmonary granulomatosis, foreign body reaction, talcosis, acute bronchospasm, pneumothorax, pneumomediastinum, pulmonary hypertension, pleuritic chest pain and blackened material in the alveolar lavage fluid [14, 15]. History taking of exposure to abused drugs often is challenging.
Epidemiology
Amiodarone, methotrexate-, nitrofurantoin-, chemotherapy agents and radiation cause lung injury with a significant incidence rate (nitrofurantoin: 0.2 %; amiodarone 2–4 % per year). Incidence with most other drugs is low to very low, fitting the numerical definition for orphan diseases of <1/2,000 patients affected. The base of evidence for drug causality is heterogenous as many reports date back several years and few specific tests were or are available. In a few phase I or II combination chemotherapy regimens where bleomycin, CCNU, gemcitabine, or dasatinib were given concomitantly with irradiation, the incidence of DIRD could be as high as 50 % or more [16].
Drugs are a significant contributor for pulmonary diseases accounting for 3–5 % of all ILD cases [17], 10 % of ARDS [18], 11–18 % of DAH [19] or eosinophilic pneumonia and 30 % of organizing pneumonia cases. Four percent of all DIRD are fatal and about 8 % are preventable although risk evaluation and prevention are still in their infancy.
Drugs cause DIRD after variable times on the medication, from a few seconds for flash pulmonary edema [20], catastrophic bronchospasm or anaphylaxis [21], to months or years into treatment for many ILD, or even after termination of treatment for iatrogenic fibrotic conditions [22]. DIRD may develop regardless of route of administration of drugs, although the oral and parenteral routes are foremost. Drugs given via topical instillation (transdermal, ophtalmic, gingival, intra-uterine, endomyometrial, subcutaneously, or in the vertebral body, pleura, urinary bladder or intrathecally), via inhalation or aspiration may also cause substantial lung injury [2].
Clinically, DIRD may manifest with involvement of the lung, central or peripheral airways, pleural and/or pericardial surface, circulation, mediastinum, neuromuscular system, heart or hemoglobin [2]. Although drugs or drug families may cause stereotyped patterns of adverse effects (Table 34.2), drugs generally may cause more than one pattern of involvement depending on the patient, with amiodarone outdoing any other drug with over 20 possible patterns [2].
Table 34.2
Main drug families capable of causing respiratory damage
Drug or family | Incidence | Involvement | Comment | |
|---|---|---|---|---|
Abused drugs/substances | Heroin, cocaine, crack, cannabis | ***** | Thermal airway injury | Crack cocaine |
Catastrophic bronchospasm | Snorted/insufflated heroin | |||
Pulmonary edema | Heroin overdose | |||
DAH | Cocaine or levamisole toxicity | |||
Pneumothorax/pyopneumothorax | Injection of drug in subclavian/jugular vein by mate | |||
Cutaneous necrotic plaques | Cocaine-levamisole toxicity | |||
Endocarditis | Intravenous drug use | |||
ACE inhibitors | Captopri, enalapril, ramipril… | ***** | Cough | Chronic. Abates with drug avoidance |
**** | Angioedema | Underdiagnosed. Ay lead to asphyxia. Relapses upon rechallenge | ||
Sartans not entirely safe | ||||
PIE | Rare | |||
Aphetamine-like anorexigens | Aminorex, fenfluramine, benfluorex | *** | PHT/Valvular heart disease | Drugs were discontinued |
Antibiotics | Minocycline, sulfasalazine, penicillin | **** | Anaphylaxis | Can cause ARF, shock, and death |
AEP | Can cause ARF. Good prognosis | |||
Anticonvulsants | Carbamazepine, phenytoin, lamotrigine | **** | DRESS | Rash, end-organ involvement, PIE in about 10 % |
Anticoagulants (oral) | Coumadin, warfarin, brodifacoum | *** | Bland DAH | Risk increases with the INR |
New direct anticoagulants (DOA) | ** | Laryngeal tongue hematoma | May cause acute UAO | |
Anticoagulants and thrombolytic agents | Heparin, SK, UK, alteplase | ** | Bland DAH | |
Hemothorax | Can cause tamponade and CV collapse | |||
Antidepressants | Sertraline, venlafaxine | AEP | ||
DRESS | ||||
Antithyroid drugs | Propylthiouracil, benzylthiouracil | *** | Capillaritis, DAH, systemic vasculitis | p- or c-ANCA present, often anti-HNE at high titers |
Renal involvement possible | ||||
Angiotensin receptor blockers | Sartans | ** | Angioedema | Risk 1/10 1/20 compared to risk of ACEI |
Beta agonists (parenteral tocolytics) | Salbutamol, terbutaline, isoxuprine | *** | NCPE | Mostly in parturients. Fatal in 5 % |
Beta-blockers | Most ß-blocking drugs | *** | Catastrophic bronchospasm | Can be fatal |
** | Lupus syndrome | Pleural/pleuropericardial effusion and a positive ANA titers | ||
* | ILD/OP | Low evidence for causality | ||
Biologics | Anti-TNF agents, rituximab, omalizumab | ** | Hypersensitivity, anaphylaxis | |
** | Acute ILD | More common with rituximab or infliximab | ||
** | Pulmonary granulomatosis | More common with etanercept | ||
** | Lupus syndrome | Serositis and high ANA and at times anti-ds-DNA | ||
Blood, blood products | Blood, blood components, FFP | **** | TRALI | Onset in 6–8 h of transfusion |
Curares | Pancuronium, tubocurarine | *** | Severe bronchospasm | |
** | Anaphylaxis | |||
Cytotoxic agents | Bleomycin, busulfan, cyclophosphamide gemcitabine, nitrosoureas, taxanes | *** | Transient pulmonary infiltrates | Caution as rechallenge may cause full-blown NCPE/ARDS |
**** | NCPE, DAH, ARDS | May relapse on rechallenge | ||
Pulmonary fibrosis | Some will benefit corticosteroid therapy | |||
Oxaliplatin | ** | Anaphylaxis | Can be fatal | |
DMARDs | NSAIDs | **** | Acute asthma. PIE | Class effect |
Methotrexate | **** | Acute cellular NSIP-like | Needs be separated from an infection and Pneumocystis pneumonia | |
Leflunomide | ** | Cellular NSIP-like | Described mostly in Japanese RA patients | |
Tacrolimus | * | ‘ILD’ | Described almost exclusively in Japanese RA patients | |
Biologics | **** | ILD/SLE/DAH | See under TNF-alpha inhibitors | |
Ergots | Bromocriptine, cabergoline ergotamine, DHE, methysergide nicergoline, pergolide | **** | Pleural effusion | |
Pleural thickening | ||||
*** | Acquired valvular heart disease | Anorexigens produced similar valvular changes | ||
Interferon alfa/beta | *** | Cellular NSIP-like ILD | New drugs to treat viral hepatitis C infection may decrease the incidence | |
OP | ||||
Sarcoid-like disease | ||||
Leukotriene receptor antagonists | Montelukast, pranlukast, zafirlukast | ** | Eosinophilic granulomatosis polyangiitis | Causal relationship needs be examined in each case |
Lipids (aspirated/inhaled) hydrocarbon | Paraffin, naphtha, kerosene | ***** | Exogenous lipoid pneumonia, HCP | Free lipids in sputum, BAL or tissue |
Lipids (infused) | Parenteral nutrition, excipients | ** | Fat embolism | |
m-TOR inhibitors | Everolimus, sirolimus, temsirolimus | **** | Cellular NSIP-like, OP, DAH | Dose-related. Abates dose with reduction or discontinuance |
Rare PAP pattern | ||||
NSAIDs, aspirin | ASA, ibuprofen, indomethacin naproxen, piroxicam | **** | Severe bronchospasm | Relapses on rechallenge |
** | PIE | Relapses on rechallenge | ||
Aspirin | *** | NCPE | Anion gap, metabolic acidosis, high salicylate levels in blood | |
Platelet GPIIb/IIIA inhibitors | Abciximab, clopidogrel, eptifibatide, ticlodipine, tirofiban | *** | DAH | |
Radiation therapy | Lung | **** | Radiation-induced lung injury | Localizes along radiation beam |
Lung | *** | Stereotactic radiation therapy | Nodule/Mass. Whorled appearance. Can be tracer-avid on PET scan | |
Mediastinum | ** | Mediastinal fibrosis | Compression of pulmonary vein | |
Trachea | ** | Stenosis | ||
Endobronchial | ** | Dehiscence | Fatal hemoptysis | |
Breast | *** | OP | Corticosteroid may be indicated | |
Liver | ** | ARDS | 131-I (radioiodine) | |
Statins | Fluvastatin, pravastatin, simvastatin | *** | Cellular NSIP-like | Ground-glass on HRCT |
OP | Fixed or migrating alveolar opacities | |||
ARDS | Statin myopathy can be present in association | |||
TKI inhibitors | Erlotinib, gefitinib | *** | DAD/ARDS | Difficult to separate from underlying disease or from an infection |
Baseline ILD may increase risk of developing the condition | ||||
Imatinib | ** | Cellular NSIP-like | Class effect of these medications | |
Dasatinib | ** | Pleural exudate, chylous effusion | ||
TNF alpha-antibody therapy | Etanercept, infliximab, adalimumab | *** | Accelerated ILD | May mimic an infection or exacerbation of underlying rheumatoid lung |
Pulmonary granulomatosis | May mimic sarcoidosis | |||
Opportunistic infections incl. TB | Pretherapy evaluation as regards latent TB indicated using TST and IGRA |
Illicit/abused drugs including alcohol (also harbored in Pneumotox) are relevant DIRD providers [2]. In the past 40 years, the death toll from opioids in the US increased nine-fold, due to ventilatory depression, falls, and aspiration [23]. Subversion of drug use, herbals, dietary supplements, incense and chemical concoctions obtained through the Internet emerge as a cause of life-threatening respiratory disease [24, 25]. The new trend of ‘Met-Labs’ may cause life-threatening injuries to the manufacturer, family, law enforcement officers, firefighters and forensic personnel [26]. Other forms of DIRD include occupational asthma in pharmaceutical industry workers, and lung injury in pets and other animals, which closely resemble those seen in humans [27–29].
Diagnosis
To raise the possibility that a new respiratory problem is caused by the administration of a drug, a combination of drugs and/or radiation therapy can be important for the patient: further/irreversible damage can be prevented if use of the harmful drug is stopped (although the underlying illness needs be managed using other medications so that the patient is not exposed to a flare of the underlying disease), patients may be spared unnecessary invasive evaluation (e.g. a lung biopsy and/or empiric corticosteroid therapy) pending the result of a drug holiday which can be diagnostic, and unnecessary readministration of the causal drug can be avoided. A high index of suspicion for drugs or chemicals as the possible cause for any new respiratory problem is warranted regardless of age as DIRD can occur in children or in newborns, gender though a few DIRD are female- or male-specific or are unique to the pregnancy state and underlying condition. Altered sensorium and inability of the patient to communicate in the emergency setting may complexify history taking, which may then rest on relatives, family physician or pharmacist. A urine drug screen and blood levels of the suspect drug or chemical (e.g. paraquat, brodifacoum) are indicated as early as possible after admission, so as the compound has not cleared to insignificant levels [30]. Analysis of the time course of symptoms vs. exposure is indicated as both drugs and patterns differ in this respect. Literature should confirm that the observed pattern (symptoms, imaging, laboratory findings, BAL, pathology, outcome) is appropriate for the specific drug. Pneumotox was designed to expedite this search [2]. Diligent exclusion of other possible causes is required as drug-induced pneumonitis and infectious pneumonia may present similarly. The expanse of differential diagnosis varies according to drug, basic disease, clinical context, presentation, pattern of injury and whether the patient was being exposed to one or several drugs or immunosuppressive agents. Abatement or resolution of symptoms should follow drug discontinuance, preferably without adjunctive corticosteroid therapy otherwise drug dechallenge is more difficult to interpret. Hyperacute drug-induced cellular ILD, pulmonary edema, alveolar hemorrhage, ARDS and systemic reactions may not improve upon simple drug withdrawal and corticosteroid therapy is indicated in such case and can be life-saving. Absence of recurrence of symptoms within an appropriate observational period off the drug will support the drug etiology. Relapse after inadvertent or deliberate rechallenge with the drug is undisputable evidence for drug causality, but this test often is not available as rechallenge can be hazardous or cause patient’s demise. Rechallenging the patient can be considered only if the drug is vital, there is no substitute for it, safe rechallenge has been described in the literature (e.g. imatinib, dasatinib, m-TOR inhibitors), and/or a prococol for rechallenge or induction of tolerance is available. Overall, 1.4 % of all DIRD cases have been rechallenged, leading to death of 7 % of those so managed. Lung pathology is rarely available in DIRD, being described in 3.8 % of all DIRD reports. Tissue abnormalities in drug-induced lung disease may be ‘consistent with’, ‘suggestive of’ or rarely ‘diagnostic of’ the drug etiology, which limits the contribution of this test. Table 34.3 lists the pathology patterns and whether BAL can be used as a surrogate test. The lung biopsy may have an important exclusionary role, whereby other illnesses or an infection can be ruled out with greater certainty. The recently described cryobiopsy technique is being currently evaluated [31]. Diagnostic criteria are summarized on Table 34.4. Overall, only a few cases can be definitely ascribed to drugs, namely those with a distinctive pathology or positive rechallenge. In a fraction, drugs can be ruled out, and the vast majority is labled possible or probable, with all uncertainty left to such wording [32]. Classic papers by Hill [33] and the Naranjo scoring system [34] still are valuable resources. The topic of counterfeit drugs [35] and the recent issue of fake journals [36] is a further challenge in evaluation the materiality of DIRD; continued vigilance is necessary.
Table 34.3
Drug-induced respiratory disease: pathology consistent with: pathology shows nonspecific findings and cannot support the diagnosis Suggestive: pathology shows distinctive findings that are distinctive enough to support the diagnosis specific: changes almost pathognomonic
Histopathologic pattern | Typical drug or drugs causing the pattern | Frequency | BAL surrogate? | Consistent with | Suggestive | Specific |
|---|---|---|---|---|---|---|
Cellular ILD, NSIP-cellular-like | Methotrexate, nitrofurantoin, sirolimus | Common | Y if lymphocytic | X | ||
Eosinophilic pneumonia | Minocycline, NSAIDs | Common | Y | X | ||
Organizing pneumonia (OP pattern) | Amiodarone, interferon | Common | X | |||
Acute Fibrinous Organizing Pneumonia AFOP | Amiodarone | Uncommon | ||||
ILD with a granulomatous component | BCG, interferon, methotrexate | Common | X | |||
ILD with a necrotizing granulomatous component | BCG, marijuana, methotrexate | Uncommon | ||||
Diffuse alveolar damage DAD | Chemotherapy, irradiation | Common | X | X | ||
A reactive epithelium, pneumocyte atypia | Alkylating chemotherapy, irradiation | Common | Y | X | X | |
Diffuse alveolar hemorrhage DAH | Anticoagulants, platelet aggregation inhibitors | Quite common | Y | X | ||
Pulmonary fibrosis, NSIP-fibrotic | Chemotherapeutic drugs | Common | ||||
Pleuroparenchymal fibroelastosis | Cyclophosphamide | Rare | ||||
UIP pattern | Chemotherapeutic drugs | Common | ||||
DIP pattern | Amiodarone, nitrofurantoin, sirolimus | Unusual | X | |||
GIP pattern | Nitrofurantoin | Rare | X | |||
LIP pattern. Lymphoid hyperplasia | Amiodarone | Unusual | X | |||
PAP pattern – secondary PAP | Busulfan, sirolimus | Rare | Y | |||
Endogenous lipoid pneumonia – phospholipidosis | Amiodarone | Very common | Y/N | X | X | |
Changes in amiodarone pulmonary toxicity can be very suggestive | Or nearly so | |||||
Exogenous lipoid pneumonia | Paraffin, mineral oil | Common | Y | X | ||
Lipid staining diagnostic | ||||||
Interstitial foreign body granuloma | Abused drugs, talc | Uncommon | Y | X | ||
Smudged parenchymal necrosis | Amiodarone | Uncommon | X | X | ||
Pneumoconiosis, talcosis | Abused drugs, talc | Uncommon | Y | X | ||
Amyloid deposits | Insulin | Rare | ||||
Crystal storage disease | Clofazimine | Rare | ||||
Diffuse pulmonary calcification | Calcium replacement | Rare | X | X | ||
Bland pulmonary edema | Chemotherapy, salicylate | Common | X | |||
Subacute/acute cellular bronchiolitis | Aspirated food, tobacco smoke, talc | Uncommon | X | X (if demonstrable food particulate matters in tissue) | ||
RB-ILD | Tobacco smoke | Common | Y? | X (if pigmented macrophages present) | ||
Constrictive obliterative bronchiolitis | ?Penicillamine ?Gold. Sauropus androgynus | Rare | ||||
Foreign body bronchiolitis | Talc | Uncommon | Y | X | ||
Pulmonary capillaritis | ATRA, PTU | Rare | X | |||
Pulmonary vasculitis other than capillaritis | Hydralazine, tryptophan | Rare | X | |||
Eosinophilic vasculitis | Tryptophan | Uncommon | Y | X | ||
Fat/marrow embolism | Parenteral nutrition, propofol, vertebroplasty | Uncommon | Y | X | ||
Silicone embolism | Fluid silicone | Uncommon | X | |||
Foreign body vasculopathy | Talc, excipients | Uncommon | X | |||
Elemental mercury embolism | Liquid mercury | Rare | X | |||
Cement embolism | Acrylate cement | Uncommon | X | |||
Crystal pulmonary embolism | i.v. lipids | Rare | X | |||
Venoocclusive disease | Antineoplastic chemotherapy | Uncommon | X | |||
Pulmonary hypertension | Anorexigens | Quite commona | X | |||
Pleuritis | Radiation therapy, drug lupus | Common | X | |||
Eosinophilic pleuritis | Propylthiouracil PTU | Uncommon | X | |||
Pleural fibrosis | Amiodarone, ergots, drug-induced lupus | Common | X | |||
Fire eater’s lung | Kerdane, petrolatum | Uncommon | X | |||
Kayexalate lung | Kayexalate | Uncommon | X | |||
Alveolar carbonaceous deposits | Crack cocaine | Uncommon | Y | X |
Table 34.4
Check list for diagnosing drug-induced respiratory disease
No evidence for pulmonary disease prior to therapy with the drug |
Event unlikely in the course of the specific disease state |
Confirmed exposure |
Eligible drug |
Drug singularity |
Absence of prodromal signs and symptoms prior to exposure to the drug |
Appropriate timing (latency time, onset) relative to taking the medication |
Pattern appropriate for the drug under scrutiny |
Confirmatory literature (qualitative: consistency, quantitative: magnitude) |
Supportive imaging, laboratory, BAL, pathological features |
Laboratory evidence including blood levels |
Reasonable exclusion of other causes including an infection and the effects of other drugs |
Abatement or resolution with discontinuation of drug, preferably without corticosteroid therapy |
Lack of recurrence is patient not rechallenged |
Relapse following rechallenge with the drug |
Rare Drug-Induced Respiratory Disease
The orphan patient is the one presenting with DIRD in whom the diagnosis is not raised and drug dechallenge is not attempted. A list of common inducers and the corresponding clinical-imaging-pathologic patterns of involvement is given in Table 34.5. Being supported by a wealthy and persistently fueled literature, involvement from these drugs is not reviewed here in great detail although atypical respiratory presentations from some of these compounds is reviewed later in this text.
Table 34.5
Drug-induced respiratory disease: common inducers and patterns of involvement
Typical clinical imaging pattern | Incidence/RR | Practical risk factors | Clinical presentation | Onset | Management | Comment | |
|---|---|---|---|---|---|---|---|
ACEI | Cough | RR 2.5 | Varies with ACEI | Chronic dry cough | w-y | DW | Resolves in all |
Angioedema | 0.1–0.4 % – RR 7.7 | Dark-skinned people | Acute UAO/Asphyxia | w-y | DW | Fatal in 5 % | |
Amiodarone | ILD, endogenous lipid PNA | Up to 4 % | Dosage, time on drug, O2 | Dyspnea, cough | d-y | Corticosteroids | Can leave residual fibrosis |
Anticoagulants, oral | DAH. Airway hematoma | Rare | INR-dependent | Acute dyspnea & anemia | w-y | DW vitK FFP | BAL diagnostic |
Anticonvulsants | DRESS syndrome | Up to 1/1,000 | Familial incidence | Skin, deep-seated involvement | mo-y | DW | Mortality ca. 5 % |
Aspirin/salicylate | Pulmonary edema | 45 % of those poisoned | Elderly | NCPE, M metabolic acidosis | h-mo | DW, alkalinization dialysis | Mortality up to 15 % |
ATRA | Pulmonary edema, DAH | 6–27 %. Less if CS given | Leukocytosis | Pulmonary edema ARDS DAH | d | Supportive + corticosteroids | Mortality up to 13 % |
Beta-blockers | Bronchospasm | Common | Asthma, atopy | Acute bronchospasm | min | Supportive + DW | 25 % fatal |
Bleomycin | Pulm infiltrates ARDS | About 10 %, up to 22 % | Retreatment, age, smoking, dose, O2 | Basilar/diffuse infiltrates | w-mo | Supportive + DW + steroids | Up to 24 % |
Chemo agents | Pulm infiltrates ARDS | Usually about 5 % | Dose, oxygen, ? CSF | Basilar/diffuse infiltrates | w-y | DW + corticosteroids | Fatality in DAD |
Cyclophosphamide | Pulm infiltrates ARDS fibrosis | Up to 12 % | Dose, oxygen | Basilar/diffuse infiltrates | w-y | DW + corticosteroids | Up to 40 % mortality |
Drugs of abuse | Pulm infiltrates, ARDS, EoP | Difficult to evaluate | Dose | Infiltrates pneumothorax burns | min-y | DW (corticosteroids) | Panoply of AEs |
PHTn | Injection of crushed tablets | PHTn | y | Treat PHT, consider Tx | UDS indicated | ||
Ergolines | Pleural thickening | Unknown | None identified | Restrictive lung dysfunction | y | DW corticosteroids rarely indicated | No fatalities. May leave residual |
Gemcitabile | Pulmonary infiltrates NCPE | 0.03–2 % | Concomitant bleomycin or irradiation | NCPE, ARDS | Within 2 mo | DW corticosteroid therapy | Mortality 20 % |
HUS | 2.2 % | NCPE, DAH, renal failure | |||||
Gold | Acute NSIP | Unknown | None identified | Diffuse infiltrates | w-y | DW + corticosteroids | Drug out of favor |
Lipid (p.o.) | Exogenous lipoid pneumonia | 15 % of institutionalized patients | Chronic aspiration | Basilar infiltrates | mo-y | DW. Corticosteroids ? | A disease of the elderly or with achalasia |
Methotrexate | Acute NSIP | 0.9–3 % | ? previously diagnosed ILD | Diffuse infiltrates | w-y | DW. Corticosteroids | Common in RA |
Minocycline | Acute eosinophilic pneumonia | Unknown | ? Atopy | Peripheral or diffuse infiltrates | w-mo | DW. Corticosteroids | Distinctive pattern |
Autoimmune ANA/ANCA disease | Unknown | Pleuropulmonary reaction | DW. Steroids. IS. Plasma exchange | When assayed ANA in 45 %; ANCA in 7 % | |||
Some patients developed PAN | |||||||
m-TOR inhibitors | Pulmonary infiltrates | Up to 35 % | Somewhat dose-related | Basilar pulmonary infiltrates. DAH | w-mo | Dose reduction. DW. Corticosteroids | Fatal cases among DAH |
ILD may equate efficacy in RCC | |||||||
Diagnostic challenge in lung Tx recipient | |||||||
Nitrofurantoin | Acute pleuropulmonary reaction | 1/5,000 | None indentified | Acute dyspnea + chest pain | d-w | DW. Corticosteroids | Some patients have ANA |
Chronic ILD or fibrosis | 1/15,000–45,000 | None indentified | Chronic dyspnea | mo-y | DW. Corticosteroids | ||
NSAIDs | Asthma, anaphylaxis | Clinical: 3 % | Atopy | Acute bronchospasm | min | O2, corticosteroids, MV | Cross reaction among COX1 inhibitor drugs |
Eosinophilic pneumonia | Higher on airway challenge | None identified | Bilateral infiltrates | mo | DW. Corticosteroids | Induction of tolerance possible | |
Nitrosoureas | Acute lung injury, ARDS | 3–5 % overall | Dose, young age | Basilar or diffuse infiltrates | w-mo | DW. Corticosteroids | A fraction will respond to steroids |
Pulmonary fibrosis | Basilar or diffuse infiltrates | mo | DW. Corticosteroids | Can be devastating | |||
Phenytoin | DRESS syndrome | 1/1,000–1/10,000 | Family history | Multiorgan | W | DW ?Corticosteroids | Other anticonvulsants may cross-react |
Radiocontrast media | Anaphylaxis | 02/100.000 PY – 1.2/million doses | Atopy | CV collapse, arrest, angioedema, shock | min | Withdrawal | |
Sulfasalazine | Eosinophilic pneumonia | Basilar or diffuse infiltrates and eosinophilia | mo | DW. Corticosteroids | Needs be separated from effect of IBD on lung | ||
TKI | DAD-ARDS | 1–5 % in Japan0.3 % outside Japan | Prior ILD | Basilar or diffuse infiltrates | d-w | DW. Corticosteroids | Fatality rate up to 40 % |
Adverse Respiratory Reactions with Indeterminate Evidence for Causality
About half the 950 drugs and agents censored in Pneumotox at this time cause ILD with a low to very low incidence rate (<10 reports overall despite many years on the market), indicating circumstantial evidence for causality. Reports are heterogenous [37], often date back many years, details were not given, patients were managed empirically with drug withdrawal and corticosteroid therapy making retrospective assessment difficult. The issue of causation vs. chance association is an unresolved one at this time and may remain so in the foreseable future. These drugs appear in Pneumotox with a 0–1 frequency [2].
Drugs Recalled or Fallen Out of Favor
Mecamylamine, hexamethonium, aminorex, tryptophan, fenfluramine, dex fenfluramine and benfluorex have all been recalled. We keep them in Pneumotox though, as any drug may regain popularity in light of a new indication as is the case with thalidomide, or because related congeners may reproduce DIRD similar to those of the parent compound.
The antneoplastic antibiotic mitomycin-C is now less commonly used than it once was. Mitomycin pneumonitis developed in about 5–10 % of the patients in the form of acute lung injury and DAD followed, in some patients by irreversible pulmonary fibrosis [38]. Corticosteroids have been effective in reversing early cases [39]. Mitomycin-associated hemolytic uremic syndrome is a rare (incidence 0.015 %) dreadful dose-related complication of mitomycin, gemcitabine and other chemotherapy regimens [40–42]. The syndrome is characterized by any association of hemolytic anemia, circulating schizocytes, thrombocytopenia, reticulocytosis, hyper- or hypotension, renal failure central nervous system symptoms, renal failure, pulmonary edema, alveolar hemorrhage, ARDS and/or pulmonary hypertension. Blood transfusions can trigger the onset of or aggravate the syndrome. Pathologic appearance is with intravascular pulmonary fibrin thrombi, pulmonary edema, hemorrhage and/or DAD. Management includes plasmapheresis, renal replacement therapy and high-dose corticosteroids. Serial monitoring of renal function and urinalysis may indicate those patients with early impending toxicity [43].
The once popular antirheumatoid drugs gold and penicillamine have mostly fallen into disuse since the advent of methotrexate, leflunomide and TNF-alfa antagonists. Severe gold-induced NSIP-like disease, obliterative bronchiolitis and Goodpasture like disease from penicillamine are for now diseases of the past [2].
Amphetamine-like anorectics (aminorex in the 1960s, fenfluramine-dexfenfluramine in the 1990s, benfluorex in the 2000s,) were recalled on the basis of acquired pulmonary hypertension (PHT) or valvular heart disease. As early as 1977, Widgren et al. reported on plexiform arterial lesions in the pulmonary circulation of young ladies who had used the anorectic aminorex [44]. In the series by Gurtner [45] prior to the advent of new therapies for PHT, death was mainly from right ventricular failure after an average of 3.5 years. Patients with higher grades of pulmonary artery pressures had worse outcomes. However, the prognosis was not as severe as that of primary PHT. Pathologically, aminorex vasculopathy is similar to primary PHT and similar findings have been reported with the use of other anorexigens [46, 47]. The aminorex epidemic peaked in the 1960s and declined in the early 1970s concomitant with aminorex discontinuation. Although household manufacture of aminorex has been responsible for sporadic PHT cases [48], most of the concern with aminorex now resides in its precursor levamisole, a veterinary antihelminthic agent that is widely used since the early 2000s to cut street cocaine [49]. Levamisole is bioconverted to aminorex in mammals [50]. One PHT case has been documented following cocaine-levamisole abuse [51], and the topic is closely monitored. Accordingly, history of exposure to cocaine and other PHT inducers is indicated in any PHT case [52]. On the other hand, levamisole can produce a distinctive p-ANCA (with dual anti-PR3 and -MPO specificity) cutaneous vasculopathy mainly involving the face, earlobes and limbs, which is covered in more detail below [12].
The potent 5-HT2B receptor antagonist anorexigen fenfluramine and its dextro-isomer dexfenfluramine have also caused a PHT epidemic with a 6.3-fold increase in risk compared to nonusers [53]. Current data from the UK and Ireland Registry indicates a 1.7 % prevalence of antecedent anorexigen use among PHT cases [54]. In addition, fenfluramine caused valvular heart disease in the form of mainly left-sided valvular retraction with regurgitation or stenosis [55]. Fenfluramine and its dextro-isomer were recalled for that reason in the late 1990s. Benfluorex was promoted as an antidiabetic drug while in fact this is a fenfluramine prodrug. Vast numbers of valvular heart disease [56] and a few PHT cases developed in France following longterm indiscriminate exposure to the drug [57]. The drug was recalled in 2009.
Illicit amphetamines remain of concern as regards ‘primary’ PHT. An undeniably strong association between stimulant use (amphetamine, methamphetamine, cocaine) and ‘idiopathic’ PHT cases has been noted in western USA [58]. Chin et al. found a history of stimulant use in 28.9 % of their ‘idiopathic’ pulmonary hypertension cases, ten-fold that in nonidiopathic PHT [58].
Several members of the thiazolinedione (glitazones) family were discontinued in some countries owing to their propensity to cause capillary leak, pleural effusions and at times irreversible heart failure [2].
L-tryptophan once was a popular neutraceutical. The compound has produced an epidemic of ‘eosinophilia-myalgia syndrome’ [59]. Blood eosinophils were increased, about half of affected patients presented with small irregular or dense radiographic opacities and pleural effusion. An ARDS picture was noted in a few [2]. Minute amounts of contaminants (denoted peak and a suffix) formed during the bioengineered-driven synthesis of tryptophan at the Showa Denko plant were blamed as the cause for the syndrome. Incident cases diminished sharply after l-tryptophan was recalled. Only rare sporadic cases are now being reported [60].
Potentially Life-Threatening Emergencies
Pulmonologists, anesthesiologists, emergency physicians, intensive care and ENT specialists, dental and other surgeons can be confronted with acute life-threatening drug-induced respiratory and/or systemic emergencies. These reactions can affect (by decreasing order of frequency) the lung, upper or lower airways, pulmonary circulation, pleura or they are systemic in nature with pulmonary involvement in association. Clinical presentations are in the form of diffuse white-out of the lung corresponding to several forms or acute lung injury or airway emergencies. The causal relationship with administration of a drug is deduced from the brief time-to-onset of the reaction upon exposure, which can be of seconds. Some reactions develop electively during the intra- or perioperative period, causing difficult diagnostic and management issues. Recognition of the drug etiology is paramount and can be life-saving.
White-Out and the Adult Respiratory Distress Syndrome (Table 34.6 and Fig. 34.1)
Table 34.6
Acute life-threatening reactions potentially causing ARDS
Clinical pathological diagnosis | Subpattern | Pathology | Typical drugs/agents | Time to onset | Tempo | BAL | Imaging (may predominate in LL) | Diagnosis | N drugs |
|---|---|---|---|---|---|---|---|---|---|
1. Pulmonary edema | Pulmonary edema | Pulmonary edema & alveolar flooding | Chemo, ARA-C, HCT, ß2+ | Short | Acute | GGO + pleural effusion | Normal heart, short tempo | 170 | |
Flash PE | ?Pulmonary edema ?DAD | Adrenaline, RCM | Ultrashort <5 min | Hyperacute | Haze, GGO, lobular thickening | Tempo, foam at mouth | 9 | ||
Transient pulmonary infiltrates | Pulmonary edema, DAD, vaculitis | Paclitaxel, ATG | Short | Acute | Slight haze, GGO, lobular thickening | Short-lived; relapse on rechalleng | 6 | ||
Pulmonary edema & shock | Pulmonary edema | Hydrochlorothiazide | Short | Acute-to-hyperacute | N | Alveolar shad ± pleural eff. | Tempo, shock, relapse on rechallenge | 2 | |
Salicylate pulmonary edema | Pulmonary edema, DAD | Salicylate | Variable | Subacute | N | Alveolar shadowing ± pleural eff. | Salicylemia, metabolic acidosis, anion gap | 1 | |
TRALI | Pulmonary edema, DAD, DAH | Blood, blood componenta, plasma | Within 8 h | Acute | N | Bilateral infiltrates or whiteout | Tempo – Relevant antibody in donor | 6 | |
2. Acute ILD | Chemotherapy lung | Pulmonary edema, NSIP, DAD, reactive pneumocytes | Chemo agents | Days-Weeks | Acute/Subacute | N + reactive cella | Haze, GGO, consolidation, whiteout | 50 | |
Acute ILD | Dense NSIP w/wo pulmonary edema or DAD | Methotrexate, nitrofurantoin, mTOR inhibitors | Variable | cute | L/N | Bilateral infiltrates | Exclusion/BAL:Pathology in selected cases | 81 | |
Acute granulomtous ILD | Acute granulomatous ILD | BCG, fludarabine, IFN, MTX | Variable | Acute | L | Bilateral infiltrates | Pathology | 24 | |
Acute foreign body reaction | Foreign body granuloma | Talc, crospovidone, food | Subacute | Subacute | – | Bilateral infiltrates | BAL, pathology | 9 | |
Acute eosinophilic pneumonia | AEP | Minocycline | Weeks | Acute | E | Bilateral infiltrates | Eos blood/BAL | 28 | |
Acute OP/AFOP | OP/AFOP w/wo foam cells | Amiodarone, statins | Variable | Acute | M | Bilateral infiltrates | Pathology | 7 | |
Accelerated pulmonary fibrosis | Dense interstitial fibrosis | Bleomycin, nitrosoureas, anti-TNF, paraquat | Days to weeks | Subacute | N | Bilateral infiltrates | Pathology | 17 | |
Acute amiodarone lung | NSIP w/wo a DIP pattern | Amiodarone | Weeks-months | Acute | M | Bilateral infiltrates | BAL/Pathology | 1 | |
Acute postoperative APT | DAD + foam cells | Amiodarone | Acute | Acute | M | Bilateral infiltrates | BAL/Pathology | 1 | |
3. DAH | Bland DAH | DAH | All anticoagulants, antiplatelet | Variable | Acute | RBC | Bilateral infiltrates | BAL | 102 |
ANA-related DAH | DAH | Hydralazine, PTU, anti TNF | Variable | Acute | RBC | Bilateral infiltrates | BAL + Ab | 3 | |
ANCA-related | DAH w/wo capillaritis | PTU, cocaine levamisole | Variable | Acute | RBC | Bilateral infiltrates | BAL + Ab | 7 | |
Secondary DAH | DAH and silicone or hyaluronate | Silicone, hyaluronate | Short | Subacute | RBC | Bilateral infiltrates | BAL, silicone, hyaluronate | 2 | |
4. Exacerbation of preexisting IPF | Subacute | NSIP/OP + fibrosis | Any drug causing ILD | Variable | Acute | L/E/N | Aggravated bilateral infiltrates | ?BAL | Many |
Precipitous | DAD + PF | Amiodarone, anti TNF, chemo | Variable | Acute | N | Bilateral infiltrates | – | 24 | |
5. Acute vasculopathy | Fat embolism syndrome | Fat embolism | Amphotericin, propofol | Short | Acute | AMa | Bilateral infiltrates | BAL | 10 |
Silicone embolism sd | Silicone embolism | Subcutaneous silicone injections | Hrs-days | Subacute/Acute | AMa | Bilateral infiltrates | BAL/Pathology | 1 | |
6. RILI | Outside the radiation field | DAD | Irradiation (chemo aggravate) | Weeks | Subacute | L | Haze, GGO | BAL, imaging | – |
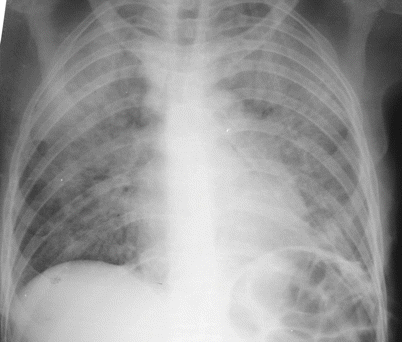
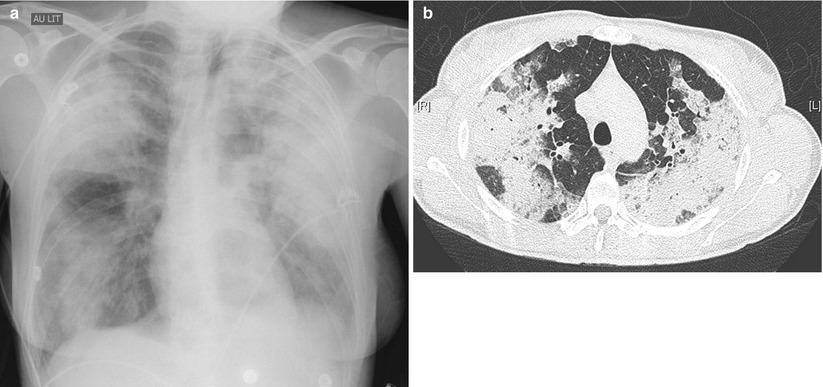
Fig. 34.1
Cellular ILD: methotrexate lung (chest tube present on lef post lung biopsy)
The Adult Respiratory Distress Syndrome (ARDS) is defined by the triad of pulmonary infiltrates in the absence of cardiac failure and a ratio of partial pressure of arterial oxygen to FIO2 of less than 300 [61]. Although this classification is relevant to the stratification of disturbances in gas exchange, it does not reflect lung pathology well. Notwithstanding the common lack of tissue available for review, the lesional pattern-based classification used by pathologists is appropriate to disentangle the group of ARDS as defined clinically and on gas exchange and has practical implications as regards the recognition, understanding and assessment of drug causality and patients’ management. Pathologically, the majority of ARDS cases show the features of diffuse alveolar damage (DAD) [62, 63]. A smaller proportion corresponds to pulmonary edema, acute- NSIP or granulomatous ILD, -eosinophilic pneumonia (AEP), organizing (OP) or -fibrinous organizing pneumonia (AFOP), or diffuse alveolar hemorrhage (DAH) with or without circulating autoantibodies (ANCA, ANA) (Table 34.6). Although pathologically distinctive, the above patterns are rarely specific for the drug etiology (Table 34.3). Diagnosis is best approached noninvasively using literature holdings, imaging features, BAL, exclusion of another cause particularly an infection and response to drug therapy withdrawal and corticosteroid therapy. Imaging is a relevant adjunct for diagnosing drug-induced lung disease and separating it from other conditions to which DILD may resemble [15, 64–74]. The chest radiograph is examined as regards vascularity, septal lines, the vascular pedicle, heart, fissures, haze, and pleural/pericardial effusion [75]. Features of interest on HRCT include haze, ground-glass, inter- or intra-lobular septal thickening, crazy-paving, pleural effusion, zonal consolidation, micronodules, lymphadenopathy, increased attenuation of lung and liver tissue, and parenchymal calcification. Data in Table 34.6 may help establish a likely diagnosis for ARDS and from this analysis, to deduce an appropriate management strategy.
Acute pulmonary edema (Fig. 34.2) can develop quickly following inadvertent or deliberate overdoses of drugs or chemicals [2] including carbamates, calcium channel blockers (nicardipine, nifedipine, verapamil), tricyclic antidepressants, abused drugs including heroin, cocaine, amphetamine and salicylate [66, 76–82]. The naloxone test and urine drug screen may be indicated in patients suspect of being overdosed with opiates. Monitoring of blood levels should be ordered in salicylate intoxicated patients since dialysis may be indicated in severely poisoned acidotic patients with salicylate levels above 100 mg/dL [30]. Drug-induced pulmonary edema (DIPE) is defined by the subacute-to-rapid onset of pulmonary infiltrates coexisting with hypoxemia and a normal cardiac function, heart ultrasound and pulmonary capillary wedge pressure if measured, in a patient receiving one of the 171 compatible drugs taken at normal dosages in the absence of other explanation. DIPE may develop following oral, parenteral or topical administration of such drugs as epinephrine, treninoin (ATRA), arsenic trioxide (As203), salicylate, CSF, hydrochlorothiazide, nafazoline, opiates, propofol (a drug with potential for addiction) [83], i.v. ß2 agonists when used as tocolytic agents near term, the chemo agents gemcitabine, methotrexate, mitomycin C or taxanes, and radiocontrast agents. Pulmonary edema can also be a complication of drug-induced anaphylaxis. Hydrochlorothiazide- and salicylate-induced pulmonary edema is diagnosed suboptimally [30, 84, 85]. The edema may develop with intake of as little as one tablet of the causal drug and the diagnosis is sometimes raised by patient or family. Abrupt onset in minutes or a few hours following exposure and rapid clearing in a few hours or days upon drug stoppage are characteristic features of DIPE. In one case, pulmonary edema developed as a consequence of radiocontrast material (RCM) administration during HRCT examination, lobular pulmonary shadowing and septal lines denoting edema were noted 25 s following drug administration [20]. Such episodes are labeled ‘flash pulmonary edema’ to reflect the extreme shortness of onset [86]. Some DIPE cases may evolve to full-blown ARDS picture after stepwise increases in edema severity with each administration of the causal drug. Severe pulmonary edema classically but exclusively so in drug abusers may be heralded by a plume of protein-rich frothy sputum at the mouth (with a protein to plasma fluid ratio >0.7) [87]. Fever, hypotension, shock and hemoconcentration concomitant with the appearance of pulmonary infiltrates characterize hydrochlorothiazide-induced pulmonary edema [88]. Instances of pulmonary infiltrates waning in a few hours have been noted after exposure to crack cocaine, hydrochlorothiazide, nitrofurantoin and anti-thymocyte globulin [2] may also correspond to drug-induced pulmonary edema for lack of better fit, although pathology is rarely available.