Radionuclide Studies of the Lung
Vicente J. Caride
Ventilation and Perfusion Studies of the Lungs
Lung perfusion scintigraphy for the diagnosis of pulmonary embolism (PE) was first reported in 1964. The perfusion study was soon complemented by a ventilation scan.51,43 Currently, the ventilation scans are performed with radiogases or 133Xe, 127Xe, 89Kr or with aerosolized 99mTc-DTPA, while the perfusion images are obtained by embolizing the lungs with 99mTc-macroaggregates of albumin (MAA). The total number of MAA particles injected should not exceed 500,000 to limit the embolization of one in every 1,000 precapillary arterioles. The number of particles should be reduced in patients with extensive lung disease, who have undergone pneumonectomy, or with severe pulmonary hypertension as well as in pediatric patients and those with known large right-to-left shunts. Reduction of radioactivity from 4 to 2 mCi (128–72 mBq) is advisable in pregnant women and pediatric patients. The macroaggregates clear the lungs with a biological half-life of 3 hours.
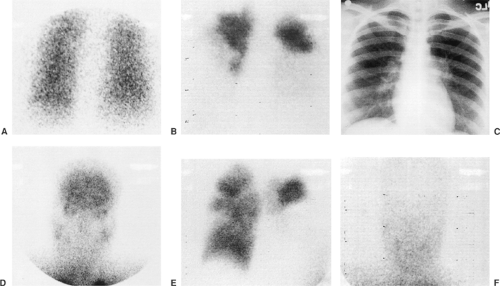
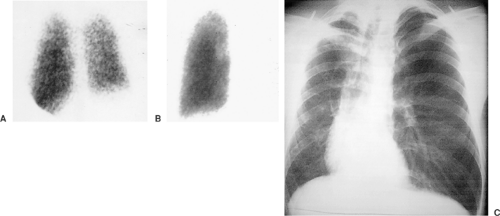
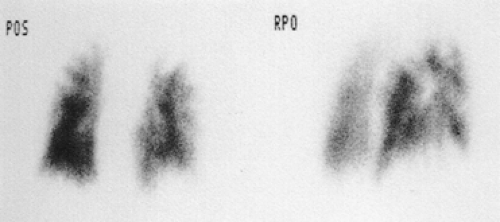
Acute and chronic pulmonary emboli (Fig. 13-1), lung tumors, radiation therapy, congenital pulmonary artery anomalies (Fig. 13-2), chronic obstructive lung disease, lymphangitic spread of cancer and tumor emboli (Fig. 13-3), fibrosing mediastinitis, vasculitis, pneumonia, bronchial obstruction, pleural effusions, and chest wall deformations result in diverse perfusion defects. Therefore, lung perfusion studies should be interpreted with knowledge of the clinical findings and compared with a ventilation scan and a chest x-ray obtained within 4 hours. The likelihood of PE is estimated by the number of perfusion defects, their size and appearance (lobar, segmental, or nonsegmental), location (peripheral or central), and whether the perfusion defects are matched by ventilation defects and chest x-ray abnormalities.
The PIOPED study44 validated the PE probability estimates based on ventilation/perfusion scintigraphy and chest x-ray findings. PIOPED confirmed that a normal perfusion study excludes pulmonary emboli with a 97% negative predictive value. A scintigraphic study showing two or more segmental perfusion defects, a normal ventilation scan, and a clear chest x-ray carries a high probability for pulmonary embolism and has a sensitivity of 41% and a specificity of 97%. In subjects with history of pulmonary emboli, a high-probability lung scan is less specific owing to the presence of unresolved perfusion defects from the previous episodes.
Nonsegmental or small perfusion defects, matched by ventilation with or without chest x-ray abnormalities larger than the perfusion defects, have a low probability for pulmonary emboli (<20% probability), while scans that do not fit into the high- or low-probability categories almost always require additional tests for diagnosing PE.
Computed tomography angiography (CTA) diagnoses lung embolism with a sensitivity of 83% (90% with CTA and CT venography) and 95% specificity. It is displacing lung scintigraphy as the first diagnostic imaging modality of choice.41 Lung scintigraphy is an alternative to CT pulmonary angiography in patients with contrast intolerance, poor renal function, individuals who are obese or claustrophobic, pediatric patients, and pregnant patients. It has been suggested that lung scintigraphy may be the first choice when there is low clinical suspicion of disease, since the perfusion lung scans are associated with very low morbidity and can select out patients with a higher likelihood of PE for CT angiography.
The triage should start with a pointed clinical evaluation to establish the likelihood of pulmonary emboli. In an attempt to provide a rational selection of patients, Wells et al.53 and later Wicki and coworkers55 identified conditions that are significant predictors for PE, including recent surgery, thromboembolic disease, history of pulmonary embolism or thromboembolic disease, malignancy, older age, hypocapnia, hypoxemia, tachycardia, and band atelectasis or elevation of the diaphragm on the chest x-ray to obtain a clinical score. A low pretest probability together with a negative D-dimer test effectively rules out acute PE.54 In populations with low prevalence of PE (<20%), scintigraphy can be used to avoid unnecessary iodinated contrast studies and select a population with a higher prevalence of PE, thus improving the positive predictive value of CTA.
Pulmonary hypertension is characterized by insidious progressive exertional dyspnea, exercise intolerance, and scant findings on physical examination. It has been estimated that 0.1% to 0.5% of patients who have survived PE may have unresolved PE. Patients whose ventilation/perfusion lung scans show peripheral, mismatched, segmental, or subsegmental perfusion defects should be further evaluated with pulmonary angiography or CT angiography to confirm the presence of unresolved emboli and to establish the central location of the pulmonary artery clots amenable for successful endarterectomy.
Occasionally there is visualization of kidneys, liver, heart, and extrapulmonary tissues after the intravenous administration of 99mTc-MAA, consistent with a right-to-left shunt. This diagnosis
is confirmed by demonstrating brain uptake of 99mTc MAA (Fig. 13-1). In patients with unexplained hypoxemia and a negative perfusion lung scan, additional images of the head should be routinely obtained to exclude right-to-left shunting of blood.
is confirmed by demonstrating brain uptake of 99mTc MAA (Fig. 13-1). In patients with unexplained hypoxemia and a negative perfusion lung scan, additional images of the head should be routinely obtained to exclude right-to-left shunting of blood.
Aerosol images are an alternative to 133Xe ventilation studies because of their simplicity and because the lungs can be imaged in multiple projections, thus facilitating the comparison with the perfusion images for PE diagnosis. For the evaluation of the integrity of the alveolocapillary membrane, the lungs are imaged continuously for 10 to 30 minutes to calculate the rate of radioaerosol clearance. Normal clearance rates have been reported from 0.5% to 2% per minute, with a half-clearance time of approximately 60 minutes. Fast clearance rates are seen in smokers and in patients with inhalation injuries, alveolitis, sarcoidosis, immunosuppression, and pneumonitis of any etiology as well as in those who have undergone radiotherapy.
Radionuclide Evaluation of Neoplastic and Nonneoplastic Lung Diseases
67Ga citrate, 201Tl chloride, and 99mTc-sestamibi are agents that accumulate in viable tumors through different mechanisms. Currently, the clinical value of these agents for the study of lung tumors is limited.
67GA Scintigraphy
After intravenous administration, the iron analog 67Ga circulates in blood mostly associated with transferrin. The cellular uptake of 67Ga is mediated by the transferrin receptors found in many normal cells and in Hodgkin’s and non–Hodgkin’s lymphomas, squamous cell and adenocarcinomas of the lung, hepatomas, adenocarcinomas, and anaplastic thyroid cancers, as reported by Tsuchiya and colleagues.46
MacMahon and coworkers26 reviewed 100 patients with lung tumors studied with 67Ga and CT and concluded that gallium scintigraphy adds little additional information to the clinical assessment or information provided by CT. In their study, gallium was superior to CT in 16% of cases. However, in most cases the lesions discovered by 67Ga were either suspected clinically (i.e., patient with neurologic symptoms and brain metastases seen on gallium, as were those with back pain and abnormal accumulation of tracer in the spine) or were located outside the area covered by the CT exam. 67Ga imaging largely missed hepatic metastases and adrenal involvement.
The clinical use of 67Ga is well documented for Hodgkin’s disease and non-Hodgkin’s lymphomas. Gallium scintigraphy adds information of tumor viability to the CT anatomic findings. Front et al.11 used 67Ga during the initial evaluation of lymphoma with the purpose of confirming that the disease is gallium-avid and also to evaluate body regions not included in standard CT examination. Gallium-positive patients can then be followed with gallium scintigraphy to evaluate response to therapy or recurrent disease.
Optimal gallium scintigraphy should include single-photon-emission computed tomography (SPECT) as well as multiple planar images of the whole body. Shortcomings of gallium scintigraphy result from the lack of disease specific uptake and from the limited spatial resolution of the study. Gallium accumulates in normal bowel, salivary glands, liver, bone, and soft tissues and in nonneoplastic conditions including granulomatous diseases, infections, iatrogenic pneumonitis, and surgical wounds. Nonspecific hilar uptake is a well-recognized false-positive finding and is largely ignored by the radiologist when the uptake is limited to and of equal intensity in both hila.
In oncology, gallium scintigraphy becomes irrelevant with the introduction of positron emission tomography (PET) 18F-fluorodeoxyglucose (18F-FDG) imaging. 67Ga citrate is not useful for diagnosing or staging lung tumors in spite of several early optimistic studies.1,8 The use of gallium when PET is not available may be justified for lymphomas and hepatomas.
Thymic rebound, usually seen in children and young adults after chemotherapy or radiotherapy, and thymic hyperplasia are also gallium-avid. In the immune-deficient patient, intercurrent infections are gallium-active. Gallium scintigraphy has been used for the early detection of Pneumocystis carinii pneumonitis and is positive in mycobacterial infections and lymphomas. Kaposi’s sarcoma is characteristically gallium-negative. In effect, a negative gallium scan in an immunosuppressed patient with abnormal chest x-ray or chest CT is almost diagnostic for Kaposi’s sarcoma. 201Tl chloride, instead, accumulates in Kaposi’s sarcoma. Lee and collaborators23 reported that a positive 201Tl scan and negative 67Ga scan favors the diagnosis of Kaposi’s sarcoma.
The use of gallium scintigraphy in the evaluation of sarcoidosis to detect active pulmonary disease has declined because of the limited diagnostic information. 201Tl and 99mTc-sestamibi, primarily used for cardiac imaging, also have affinity for neoplastic tissues and some other nonneoplastic conditions. 201Tl-sestamibi, a potassium analog, distributes in tissues following regional blood flow and is taken up by cells via the Na, K-ATPase pump. Therefore, accumulation of this tracer reflects cell viability and regional blood perfusion. Breast, lung, and differentiated thyroid cancer, thymomas, lymphomas, some bone tumors as well as parathyroid adenomas and sarcoidosis have been reported as 201Tl-avid lesions. Thallium, in contrast to gallium, accumulates in viable tumors with minimal uptake in inflammatory and necrotic tissue.
Higashi and coworkers14 compared 201Tl with 18F-FDG PET and found a similar detection rate and similar differentiation of benign from malignant lung nodules in lesions >0.8 cm. False negatives, however, are expected with small lesions and in
lesions located near the heart or lung bases. The authors suggest that 201Tl may be better than 18F-FDG in detecting slow-growing bronchioloalveolar tumors, although the difference was not statistically significant. If 18F-FDG PET is not available, the use of 201Tl to evaluate lung nodules in patients who refuse biopsy or are at risk for complications may be justified. A lung nodule that is positive with 201Tl should be biopsied or resected. Negative nodules or nodules that, because of their location or size, cannot be optimally evaluated should be followed clinically and with CT.
lesions located near the heart or lung bases. The authors suggest that 201Tl may be better than 18F-FDG in detecting slow-growing bronchioloalveolar tumors, although the difference was not statistically significant. If 18F-FDG PET is not available, the use of 201Tl to evaluate lung nodules in patients who refuse biopsy or are at risk for complications may be justified. A lung nodule that is positive with 201Tl should be biopsied or resected. Negative nodules or nodules that, because of their location or size, cannot be optimally evaluated should be followed clinically and with CT.
99mTc-sestamibi is a lipophilic cation that passively accumulates in the cytoplasm and mitochondria, driven by differential transmembrane potentials, as reported by Maublant et al.27 99mTc-sestamibi is a substrate for the transmembrane p-glycoprotein (Pgp) encoded in the multidrug-resistance (MDR1)gene. The Pgp is found in adrenal glands, kidneys, luminal epithelium of colon, and sometimes the lungs. It is overexpressed in certain tumor cell lines and is responsible for the resistance to chemotherapy of tumors. This characteristic of 99mTc-sestamibi received the attention of researchers hoping to develop a test to identify patients whose tumors were resistant to chemotherapeutic drugs that are also substrate of Pgp. A multidrug resistance–associated protein (MRP) was demonstrated in cells that do not express the MDR1 gene. The MRP is expressed in lung, testis, peripheral mononuclear cells, and SCLC as well as in NSCLC resistant cell lines.
Zhou and colleagues57 studied 34 lung cancer patients with 99mTc-sestamibi SPECT and after surgery analyzed the tumors by immunohistochemistry to assess the expression of Pgp, multidrug resistance–associated protein (MRP), and lung resistance protein (LRP). The washout rate of 99mTc-sestamibi in tumors expressing Pgp was higher than in the Pgp-negative tumors. The authors did not find a relation between the washout rate of 99mTc-sestamibi and the expression of MRP or LRP.
Clinical studies by Dirlik and collaborators9 failed to provide definitive evidence that imaging studies could select out patients whose lung tumors expressed the MDR1 gene. This failure is expected if SCLC does not express the transmembrane glycoprotein whose substrate is 99mTc-sestamibi. 99mTc-sestamibi has limited use in evaluating patients with lung cancer, and at this time the potential of this agent to select patients for alternative therapies has not been demonstrated.
Parathyroid adenomas can be readily identified using 201Tl or 99mTc-sestamibi scintigraphy. While most parathyroid adenomas are localized during surgery without the aid of imaging, preoperative localization of the lesion reduces the length of the procedure and on occasion detects adenomas in aberrant location. It also allows the selection of patients for selective surgical intervention. Preoperative parathyroid imaging is advantageous for patients who experienced a recurrence of hyperparathyroidism after parathyroidectomy.
The study can be performed with SPECT by obtaining images of the neck and mediastinum 15 minutes and 2 hours postinjection. Subtraction images using 123I thyroid imaging are optional. Normal thyroid tissue and functioning parathyroid adenomas take up 99mTc-sestamibi during the immediate postinjection period. Parathyroid adenomas retain higher levels of activity owing to higher blood flow and higher metabolic activity than the surrounding tissues and are visible on the delayed images. The procedure, however, is not as accurate in diagnosing parathyroid hyperplasia.
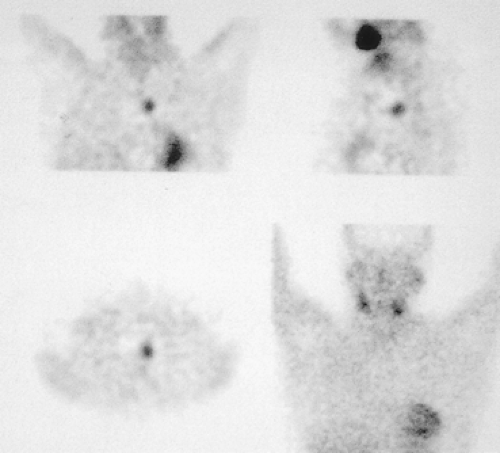 Figure 13-4. Single-photon-emission tomography with 99mTc-sestamibi allows more precise location of a mediastinal parathyroid adenoma. |
Scintigraphy cannot determine whether the adenoma originates from the superior or inferior parathyroid gland, but it will indicate the adenoma’s location; SPECT provides better localization of mediastinal adenomas and their relation to the thyroid, trachea, and esophagus (Fig. 13-4). Gamma handheld probes during surgery can be useful in recurrent hyperparathyroidism and for small lesions whose location is unclear. Routine imaging of transplanted parathyroid tissue is important, since transplantation occasionally is responsible for recurrent hyperparathyroidism. 99mTc-sestamibi accumulates in benign and malignant thyroid nodules, lung tumors, metastatic lymph nodes, sarcoid, and other active granulomas. Extravasated 99mTc-sestamibi at the injection site results in the visualization of normal axillary lymph nodes. Persistent tracer uptake along the vein after the injection is a common occurrence and, in some cases, mimics a focal mediastinal abnormality. In this situation the study should be repeated, injecting the radiotracer through a foot vein to rule out a lesion.
Failure to detect parathyroid adenomas is likely with subcentimeter lesions located near radioactive structures or with suboptimal studies. Kao and coworkers22 evaluated 47 patients with parathyroid adenomas >1.5 g. In eight instances, 99mTc-sestamibi scintigraphy failed to detect parathyroid adenomas. All the nonvisualized adenomas expressed Pgp or MRP, responsible for the extrusion of 99mTc -sestamibi. The other 39 detected adenomas did not express Pgp or MRP.
Somatostatin Receptor Scintigraphy
Somatostatin (SST), a 14– or 28–amino acid peptide—found in the hypothalamus, cerebral cortex, brainstem, gastrointestinal tract, and pancreas—is a neurotransmitter in the central nervous system (CNS) and gastrointestinal tract with inhibitory hormonal actions on growth hormone, insulin, glucagon, gastrin, serotonin, and calcitonin. SST has an antiproliferative
effect on tumors by inhibiting growth and angiogenesis and the release of growth factors and hormones. It also has an inhibitory effect on inflammatory cells and acts as an immunomodulator. There are at least 5 SST receptor subtypes variously expressed in normal tissues and neuroendocrine tumors. These receptors are also present in activated lymphocytes, lymphomas, and other nonneuroendocrine tumors in the brain, breast, and lungs.
effect on tumors by inhibiting growth and angiogenesis and the release of growth factors and hormones. It also has an inhibitory effect on inflammatory cells and acts as an immunomodulator. There are at least 5 SST receptor subtypes variously expressed in normal tissues and neuroendocrine tumors. These receptors are also present in activated lymphocytes, lymphomas, and other nonneuroendocrine tumors in the brain, breast, and lungs.
Octreotide is an 8–amino acid analog of SST that has affinity for SSTR subtype 2 and less so for subtypes 3 and 5 and no significant affinity for types 1 and 4. A radiolabeled form of octreotide, 111In-octreotide, is used for imaging of neuroendocrine tumors. 111In-octreotide planar and SPECT scintigraphy is performed at 4, 24, and 48 hours after administration. The tracer accumulates in organs that express SSTR, including the pituitary, thyroid, spleen and in excretory organs including the liver, kidneys, and bladder. Octreotide is partially reabsorbed in the kidneys explaining the often obtrusive intense persistent renal activity. The hepatobiliary excretion results in bowel activity and gallbladder visualization. Nonspecific activity of 111In-octreotide is seen in wounds, healing surgical incisions, and after radiation therapy.
Hodgkin’s and non-Hodgkin’s lymphomas as well as breast and thyroid cancers and sarcomas express STT receptors and are detectable with radioactive octreotide. Reubi et al.42 investigated the expression of SST and vasoactive intestinal peptide (VIP) receptors in mesenchymal tumors. SST receptors were consistently found in cells derived from bone and vessels, particularly highly cellular, poorly differentiated osteosarcomas, but not in osteoid-forming highly differentiated tumors. SST receptors are also expressed in angiosarcomas and hemangiopericytomas, some muscle tumors, and synovial sarcomas. No SST receptors were found in chondrosarcoma, Ewing’s sarcoma, liposarcoma, mesothelioma, schwannoma, and Wilms’ tumors. VIP receptors were present in liposarcomas, mesotheliomas, and Wilms’ tumors as well as in muscle tumors and synovial sarcomas but not in osteosarcomas. Granulomatous and autoimmune conditions express SST receptors and are detectable by SST analog scintigraphy, accounting for false-positive results in the investigation of malignancies. Shorr et al.40 investigated the use of the radiolabeled SSTe analog 99mTc-depreotide in 22 patients with sarcoidosis. Depreotide provides superior images than those of 67Ga-gallium citrate and allows same day imaging. However, the scintigraphic study does not affect the current diagnostic strategies for sarcoidosis.
Glioblastomas, NSCLC, exocrine pancreatic tumors, and squamous cell cancer are tumors that lack SST receptors and yet may take up 111In-octreotide. Kwekkeboom and colleagues21 studied 40 NSCLC patients with positive 111In -octreotide scintigraphy and explained the uptake by activated lymphocytes and leukocytes around the tumor.
Lung tumors with neuroendocrine characteristics include typical and atypical carcinoids (low- and intermediate-grade carcinomas), large cell carcinomas (high-grade carcinomas) and small cell carcinoma (SCLC) can be located, diagnosed, staged, and therapy planned with octreotide imaging (Fig. 13-5). While the tumors are often treated surgically and with chemotherapy or radiotherapy, therapy with octreotide and other SST analogs is becoming more frequent for advanced disease. The demonstration of STT receptors by imaging is, therefore, important before considering therapy with radioactive STT analogs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree