Radiologic Evaluation of the Esophagus
Richard M. Gore
Yahid Yaghmai
Gary G. Ghahremani
The principal method of diagnosing functional or structural abnormalities of the esophagus is fluoroscopic evaluation combined with digital radiography while the patient is drinking a suspension of barium sulfate. This is a simple, cost-effective technique for demonstrating esophageal lesions that typically manifest with dysphagia or odynophagia. Not infrequently, however, the existence of an esophageal disorder is first appreciated during radiologic studies for unrelated or nonspecific clinical symptoms. Maglinte and associates34 detected unsuspected esophageal disease in 76 of 500 (15%) consecutive patients who were referred for upper gastrointestinal series because of abdominal pain or digestive complaints. Therefore most radiologists consider it a prudent practice to routinely survey the entire esophagus as an integral part of any barium examination of the upper gastrointestinal tract.
In addition to the conventional barium esophagography, several other imaging techniques are currently available for evaluation of the esophagus. The selection of an appropriate examination requires a tailored approach based on clinical information about the character and duration of symptoms, their relationships to coexistent systemic diseases, or any previous surgical, diagnostic, or therapeutic procedures involving the esophagus. This chapter provides an overview of the radiologic modalities used for the demonstration and differential diagnosis of various functional or organic disorders affecting the esophagus. A discussion of the noninvasive staging of squamous cell and adenocarcinoma of the esophagus is then presented.
Radiographic Methods of Examination
Plain Chest Radiography
The esophageal body contained within the posterior mediastinum has a muscular wall that is 3 to 5 mm thick, but it is normally invisible on plain films of the chest. This is partly because the esophagus collapses during its resting phase, whereas the upper and lower esophageal sphincters maintain their tonic contraction to prevent aspiration of air and retrograde flow of gastric contents into the esophagus. Nevertheless, the standard posteroanterior and lateral chest radiographs may provide significant clues to the diagnosis of an underlying pathology when the esophageal lumen contains an abnormal collection of air, fluid, or food particles or harbors an ingested radiopaque object. Furthermore, chest films may show a retrocardiac soft tissue density with an air–fluid level and thereby suggest an otherwise unsuspected hiatal hernia, as noted by Stark and associates.54
The term air esophagogram denotes the plain film visualization of an atonic, noncollapsing esophagus by virtue of retained air in its lumen. This finding was initially observed in patients with esophageal involvement by scleroderma. However, the same phenomenon may occur with mediastinal fibrosis secondary to inflammation or irradiation, after thoracic surgery, or when esophageal motility and intraluminal pressure are disturbed by
pulmonary infiltrates, which cause diminished compliance of the lungs and poor respiratory excursion.
pulmonary infiltrates, which cause diminished compliance of the lungs and poor respiratory excursion.
On chest radiography, diffuse mediastinal widening in the absence of a normal gas collection in the gastric fundus might be a manifestation of a dilated esophagus, usually containing a foamy mixture of air, food, or secretions. These features are highly suggestive of achalasia but may also be seen with a peptic stricture or infiltrating tumor of the gastroesophageal junction. Carcinoma of the esophagus may also be visible on chest films as a soft tissue mass thickening the esophagopleural stripe, indenting the trachea, or causing mediastinal adenopathy and pulmonary metastasis. Furthermore, plain radiographs of the chest and neck play an important role in the diagnosis of ingested foreign bodies and suspected esophageal perforation, as emphasized by one of us (G.G.G.).15,17
Single-Contrast Esophagram with Barium
The standard contrast material for opacification of the esophageal lumen is a colloidal suspension of micropulverized barium sulfate in water. Commercial products of various densities and viscosities are available. They usually contain flavoring agents as well as chemical additives such as aluminum hydroxide, sorbitol, and methylcellulose.
Radiologic examination of the esophagus is performed under fluoroscopic observation. In conventional single-contrast esophagography, the patient is first examined in the upright and then in the recumbent position while taking sequential swallows of a relatively dilute mixture of barium sulfate in water (40% to 80% weight per volume). Images of the well-distended esophagus are obtained, including views of the gastroesophageal segment during suspended respiration, which accentuates any existing hiatal hernia or Schatzki’s ring. In infants, a nursing bottle is used for oral administration of nonionic, isotonic iodinated contrast material, but controlled instillation through a soft feeding tube is recommended if esophageal atresia or tracheoesophageal fistula is suspected.
The uniform tubular shape of the opacified esophageal lumen and the repetitive nature of its peristaltic activity permit an accurate fluoroscopic analysis of both motility and structural changes. In addition, a permanent digital record of the observed normal or pathologic findings is routinely obtained. Continuous dynamic recording is the preferred technique for the evaluation of pharyngeal and esophageal motility. Fluoroscopic study of esophageal peristalsis and motility disorders is conducted with the patient in the prone-oblique position. The horizontal placement eliminates the interference of gravity with the passage of contrast material and permits better visibility of the esophagus by projecting it away from the thoracic spine. The patient is instructed to swallow one mouthful of barium at a time. The initiated primary peristaltic wave appears as a lumen-obliterating contraction that propagates distally at 2 to 4 cm per second. In normal adults, the bolus transit through the 20- to 24-cm long esophagus is completed in 6 to 8 seconds. Patients with esophageal dysmotility, however, usually show considerable retention and delayed clearing of barium. The associated fluoroscopic findings are decreased incidence and amplitude of peristaltic waves after deglutition, failure of the initiated contraction to progress distally, and repetitive nonpropulsive waves, commonly referred to as tertiary contractions. Ott44 and Aksglaede and coworkers2 have reported that in contrast to the dilated atonic esophageal body in achalasia, both the entity of nutcracker esophagus and diffuse esophageal spasm are characterized by recurrent high-amplitude contractions that cause dysphagia and cramping retrosternal pain. In this context it should also be noted, as pointed out by Ott45 and Richter49 and their associates, that functional and organic abnormalities of the esophagus are common sources of noncardiac chest pain, and barium esophagraphy can provide the correct diagnosis.
Esophagram with Water-Soluble Iodinated Contrast Media
Iodinated water-soluble preparations—such as Gastrograffin (Bracco Diagnostics, Princeton, NJ) or Hypaque (Amersham Health, Princeton, NJ)—have been used in instances of suspected esophageal perforation and anastomotic leakage. These aqueous contrast media are readily absorbed after extravasation into the mediastinal soft tissues and pleural or peritoneal spaces, but they rarely define the anatomy of the defect clearly. As documented by Foley14 and Buecker7 and their colleagues, the mucosal tears and transmural perforations of the esophagus are better diagnosed with barium than with iodinated contrast media. These researchers have pointed out that 25% to 50% of esophageal perforations are unrecognizable or inadequately shown during initial evaluation with water-soluble agents because their low density and rapid diffusion into the surrounding tissues impairs an optimal mucosal coating and visualization of extraluminal leakage. Furthermore, the aspiration of such iodinated hypertonic solutions into the lungs can lead to pulmonary edema and chemical pneumonitis, particularly among patients with esophageal dysmotility or obstruction. Brick and associates5 as well as one of us (G.G.G.)16 have pointed out that nonionic low-osmolality contrast media—such as Omnipaque (Amersham Health, Princeton, NJ) or Isovue (Bracco Diagnostics, Princeton, NJ)—may be used safely. Much more recently, the use of CT oral contrast esophagraphy followed by an esophagraphic chest CT has made their argument moot.
Double-Contrast Esophagography
Double-contrast esophagography permits an accurate demonstration of mucosal abnormalities that are usually the hallmark of inflammatory or neoplastic processes. The examination requires specially formulated high-density barium for coating the esophageal mucosa while the lumen is fully distended by gas. For this purpose, carbon dioxide released by ingested effervescent agents, such as citrocarbonate granules, is used together with swallowed air. This serves as a radiolucent intraluminal gas collection to expand the lumen and provide a detailed view of the esophageal inner surface. Any areas of narrowing or rigidity are also better recognized when the otherwise pliable esophageal wall is maximally stretched.
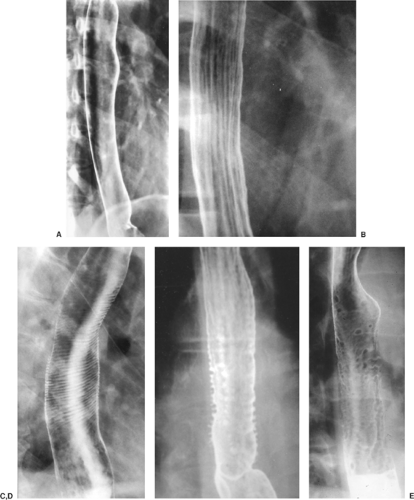
Double-contrast radiography of the normal esophagus (Fig. 133-1A) typically shows a smooth, featureless mucosa and the well-demarcated walls of this tubular structure. The longitudinal folds of the esophagus become visible when the esophagus
is collapsed or when there is mild esophagitis of the distended esophagus (Fig. 133-1B). Transverse striations (Fig. 133-1C) of the esophagus, the so-called feline esophagus, are seen in patients with reflux disease; this is caused by contraction of the longitudinally oriented muscularis mucosae. Some patients develop dilated submucosal glands, and when these fill with barium, they appear as multiple small outpouchings on esophagrams (Fig. 133-1D), simulating ulcerations.
is collapsed or when there is mild esophagitis of the distended esophagus (Fig. 133-1B). Transverse striations (Fig. 133-1C) of the esophagus, the so-called feline esophagus, are seen in patients with reflux disease; this is caused by contraction of the longitudinally oriented muscularis mucosae. Some patients develop dilated submucosal glands, and when these fill with barium, they appear as multiple small outpouchings on esophagrams (Fig. 133-1D), simulating ulcerations.
In approximately 30% of patients >50 years of age, however, a finely nodular surface pattern may be present (Fig. 133-1E) because of glycogenic acanthosis of the esophagus. This benign asymptomatic condition is characterized by glycogen deposits within multifocal plaques of hyperplastic squamous epithelium.
Multiple longitudinal folds are commonly visible in a partially collapsed esophagus, but they become totally effaced and obliterated with progressive luminal distention. Hence the detection of prominent thickened folds on air-contrast views indicates their loss of pliability due to submucosal edema and diffuse inflammatory changes. Superficial ulcerations and extensive mucosal irregularities are found in severe esophagitis caused by reflux (Fig. 133-2), caustic injury, and infections, such as candidiasis.
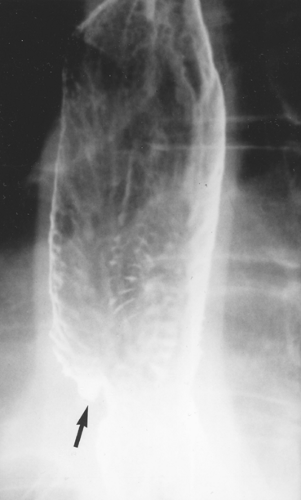 Figure 133-2. Reflux esophagitis with ulcer (arrow) and “stepladder” appearance with multiple transverse folds in the distal esophagus due to scarring. |
The classic radiologic features of Barrett’s esophagus are a high esophageal stricture or ulcer (Fig. 133-3A) associated with a sliding hiatal hernia, gastroesophageal reflux, or both. A reticular pattern characterized by innumerable tiny barium-filled grooves or crevices on the esophageal mucosa can also be seen (Fig. 133-3B).
Solid-Bolus Test
Subtle areas of narrowing and symptomatic lower esophageal rings can be evaluated by the use of commercially available barium tablets 12.5 mm in diameter or marshmallows of predetermined size. Ott and colleagues46 and one of us (G.G.G.) and colleagues18 have reported that this permits more accurate measurement of the narrowed lumen and its functional significance.
Evaluation of the Esophagus by Other Imaging Techniques
Computed Tomography
The esophagus is usually well delineated by computed tomography (CT). The paraesophageal fat is visualized as an interface between the esophagus and adjacent vascular, cardiac, and connective tissue structures. The cervical esophagus lies near the midline, posterior to and occasionally indenting the trachea. It usually does not contain air. As the esophagus enters the thorax, it lies posterior and slightly to the left of the trachea, and the esophagotracheal fat pad is often thin. More inferiorly, the esophagus is situated close to the posterior surface of the left mainstem bronchus, descending thoracic aorta, and thoracic spine. Tumors, adenopathy, and aneurysms can all displace and invade the esophagus.
Small amounts of intraesophageal air are seen in approximately 65% of normal individuals. Megibow37 recorded that the presence of an air–fluid level, fluid-filled lumen, or lumen caliber >10 mm usually indicates obstruction or severe esophageal dysmotility. Although there are no normal standards for the cross-sectional diameter of the collapsed esophagus, the wall thickness of a distended esophagus should not exceed 3 mm.
As the esophagus becomes intra-abdominal, it courses anteriorly and to the left to enter the stomach at the gastresophageal junction. This region is often problematic, because in nearly one-third of normal individuals, prominent soft tissue in this region projects into the gastric lumen. This soft tissue “mass” represents the combined thickness of the esophageal walls and medial gastric wall with its nondistended mucosal folds. A hiatal hernia can create a similar appearance. Rescanning the patient in the left lateral decubitus position with more contrast medium or air usually resolves the problem.
Hiatal hernias are manifested by widening of the esophageal hiatus associated with separation of the diaphragmatic crura and an increased distance between the crura and the esophageal wall.
The value of CT in diagnosing various other esophageal disorders has been well described in the radiologic literature. For example, varices in the esophageal wall or periesophageal region are visible on CT in 85% of endoscopically proven cases. Particularly useful, however, as pointed out by one of us (G.G.G.)17 and Noh and coworkers,42 is CT evaluation of accidental or iatrogenic perforations of the esophagus.
Esophageal Endosonography
In performing esophageal endosonography, it is vital to understand the normal sonographic anatomy of the gut and adjacent structures. Sonographically, the normal esophagus is divided into three parts: upper, middle, and lower. The upper portion extends from the oropharynx to the superior region of the aortic arch. The great vessels can be imaged as they emerge from the aortic arch and extend to the neck. The middle portion extends from the aortic arch to the subcarinal region, where the aortic arch and descending aorta project posteriorly. The distal portion extends from the subcarinal region to the level of the cardia. The left atrium projects anteriorly and the descending aorta posteriorly.
Mallery and Van Dam35 and Richards and associates48 have described the normal appearance and makeup of the wall of the esophagus as seen on the sonogram. The esophageal wall is stratified into five layers sonographically. The first layer is hyperechoic and corresponds to the superficial mucosa. The second layer is hypoechoic and corresponds to the deep mucosa. The third layer is hyperechoic and corresponds to the submucosa and the interface between the submucosa and the muscularis propria. The fourth, hypoechoic layer corresponds to the muscularis propria, and the fifth layer corresponds to the adventitia.
Magnetic Resonance Imaging
Esophageal magnetic resonance imaging (MRI) is compromised by respiratory and cardiac motion. The esophagus is most often
imaged in the body coil because the respiratory artifacts produced by a surface coil positioned on the rib cage can be difficult to eliminate. Respiratory artifacts can be minimized by respiratory compensation and single-breath-hold pulse sequences. In addition, cardiac gating and gradient moment nulling help reduce artifacts from great vessel and cardiac pulsation. Spatially selective presaturation pulses on spin-echo sequences also minimize artifacts from flowing blood, as Semelka and coworkers53 noted in Semelka’s textbook Abdominal and Pelvic MRI.
imaged in the body coil because the respiratory artifacts produced by a surface coil positioned on the rib cage can be difficult to eliminate. Respiratory artifacts can be minimized by respiratory compensation and single-breath-hold pulse sequences. In addition, cardiac gating and gradient moment nulling help reduce artifacts from great vessel and cardiac pulsation. Spatially selective presaturation pulses on spin-echo sequences also minimize artifacts from flowing blood, as Semelka and coworkers53 noted in Semelka’s textbook Abdominal and Pelvic MRI.
The esophagus appears as a low-signal-intensity structure contrasted by high-signal-intensity fat on T1-weighted images. On T2-weighted images, the muscular wall of the esophagus has low signal intensity, whereas intraluminal contents have high signal intensity. According to Semelka and colleagues,53 the muscular wall of the esophagus enhances moderately after the injection of intravenous gadolinium diethylenetriaminepenta-acetic acid (Gd-DTPA).
Although not a primary means of imaging the esophagus, MRI can serve as an alternative technique to CT and represents an important supplemental imaging method.
Positron Emission Tomography
Developed nearly three decades ago for research purposes, positron emission tomography (PET) evolved into a powerful clinical imaging tool for evaluating oncology patients. Cancers of the lung, colorectum, breast, brain, head, and neck, as well as lymphoma and melanoma, have proven to be effectively imaged and staged by PET. The efficacy of PET in cancer of the esophagus has been established by the studies of Antoch and associates.3 In vivo PET imaging in malignancies is based on the fact that the uptake of glucose and hence fluorine-18 (18F) fluorodeoxyglucose (FDG) is facilitated by two factors: the increased number of glucose transporters on the surfaces of malignant cells and a block in the intracellular glycolytic pathway due to relative glucose-6-phosphatase deficiency. PET images reflect the relative stage of glycolysis, which is increased in tumor cells. Tumors with the highest metabolic rates demonstrate the greatest accumulation of FDG.
Positron Emission Tomography/Computed Tomography
Recent technical advances have led to the development of combined PET and CT scanners. This fusion of morphologic (CT) and functional (PET) data dramatically improves the diagnostic potential of either scanner alone. Having both imaging technologies in a single machine allows for more precise localization of metabolic abnormalities. Indeed, PET/CT will become an initial staging procedure for tumors if and when reimbursement issues are resolved, as noted by Antoch and coworkers.3
Scintigraphy
Gastroesophageal reflux can be detected and quantitatively analyzed by scintigraphy after oral administration of technetium-99m (99mTc) sulfur colloid mixed in 300 to 500 mL of acidified orange juice. This technique is also useful for the evaluation of esophageal motility disorders and the postoperative evaluation of esophageal transit time, as reported by Datz.9
Angiography and Interventional Radiology
The multiplicity of sources for arterial and venous circulation of the esophagus has limited the application of angiogra- phic methods to this organ. As recently reviewed by Nemcek and Vogelzang,41 selective catheterization of the left gastric or splenic arteries can demonstrate the site of hemorrhage from esophageal varices or Mallory-Weiss tears. Such bleeding may also be treated by transcatheter infusion of vasoconstrictive agents and controlled embolization. Percutaneous transhepatic catheterization of the portal vein and its branches has been widely used for embolotherapy of bleeding gastroesophageal varices.
Transjugular intrahepatic portosystemic shunts (TIPS) are quite useful in treating recurrent variceal hemorrhage that does not respond to endoscopic sclerotherapy, variceal banding, or systemic vasopressin. TIPS are also useful in the setting of intractable ascites and portal gastropathy.
Several interventional techniques that were initially designed for the cardiovascular system have been modified for application to the alimentary tract. For instance, angioplasty balloon catheters are now being used to dilate esophageal strictures under fluoroscopic guidance. This procedure and its clinical results have been reviewed by Therasse and associates.56 Abdal and coworkers1 used this technique successfully to treat postsurgical esophageal strictures. Intravascular stents also have been modified by Lee31 for fluoroscopically controlled intra- esophageal placement. These endoluminal stents provide an effective means for palliating dysphagia caused by obstructing tumors and blockage due to esophagobronchial fistulas, both having been reported by O’Donnell43 and Roy-Choudhury51 and their colleagues.
Radiologic Diagnosis of Esophageal Cancer
Barium Studies
The esophagram has a high sensitivity in the diagnosis of eso- phageal cancer and accurately predicts lesion length in 59% of patients, according to Drudi and collaborators.10 Polypoid and stenotic tumors are more easily seen than flat, sessile lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree