Radiation Therapy for Carcinoma of the Lung
Ritsuko Komaki
James D. Cox
Carcinoma of the lung encompasses a group of diseases that require the collaboration of the thoracic surgeon, radiation oncologist, medical oncologist, pathologist, and diagnostic radiologist. Unless dramatic gains in prevention are made in the next decade, the effective interplay of these specialists represents the best hope for the millions of patients worldwide who will develop cancer of the lung.
Radiation therapy, like surgical resection, is a technically complex locoregional treatment that can be used with curative intent. The results depend on the experience of the physician-led team and the appropriate application of the treatment—that is, to those patients with disease confined to a primary tumor and regional lymph node metastasis. For patients with disease too advanced to be considered potentially curable, radiation therapy can also be used to relieve symptoms from the intrathoracic tumor and discrete metastases, especially in the brain or bones.
The endpoints used to evaluate the effectiveness of surgery, radiation therapy, and chemotherapy differ. In surgical management of lung cancer, the focus is on curability and the endpoint is survival. The most common endpoints used in evaluating chemotherapy for lung cancer are complete and partial response rates and median survival. Radiotherapeutic effectiveness may be assessed in terms of both sets of endpoints. The palliative contribution may be reported in terms of response rates and median survival. Median survival is not necessarily related to long-term survival. The lack of sensitive indicators of recurrence after radiation therapy, however, and the recognition that the treatment can cause changes in the irradiated volume that obscure response, raise questions as to the value of response as a useful endpoint. Metabolic response as measured by modalities such as positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG), however, may be an important prognostic indicator of prolonged survival after radiation therapy.10
Standard treatments for most patients with carcinoma of the lung without distant metastasis have changed in recent years. Integration of two or all three therapeutic modalities rather than use of only a single modality is advantageous for most patients. Combinations of resection and irradiation improve local tumor control and may reduce the risk of distant metastasis. Chemotherapy has increasingly been found to be effective in eradicating subclinical metastases; it also may improve local control if used concurrently with radiation therapy.
One of the challenges in treating lung cancer is proper application of the treatments to those patients most likely to benefit. For patients with inoperable squamous cell carcinoma, adenocarcinoma, or large-cell (non-small-cell) lung cancer (NSCLC), pretreatment prognostic variables probably outweigh treatment factors in their short-term survival. At least 20% of all patients with NSCLC have unresectable tumors without distant metastasis, little loss of weight (<5%), and mild to moderate symptoms (Karnofsky performance status scores of 70 to 100). One of us (R.K.) and associates36 showed that disease in such patients was potentially curable with radiation therapy alone. However, the outlook for patients with relatively favorable but inoperable disease is better with induction chemotherapy followed by radiation therapy, as demonstrated by Dillman,23 Le Chevalier,42 and Sause61 and their colleagues. Chemotherapy and radiation therapy administered concurrently increase the probability of survival.19,27
Small-cell carcinoma, heretofore considered the most malignant form of cancer of the lung, is more likely to be cured than NSCLC when the tumor is limited to the primary site and regional lymph nodes. Radiation therapy to these regions, given concurrently with combination chemotherapy, has been shown by Turrisi and coworkers71 to cure more than 25% of patients with limited small-cell carcinoma.
Indications for Radiation Therapy Based on Failure Analyses
Shields’s64 failure-pattern analyses of patients with resectable carcinoma of the lung suggested that a subset of patients with squamous cell carcinoma, adenocarcinoma, or large-cell carcinoma of the lung could benefit from irradiation of the mediastinum and hila. Patients with T1N0M0 or T2N0M0 disease rarely experience failure in the thorax after complete resection of the tumor. The presence of mediastinal lymph node metastasis considerably increases the risk of local failure and also portends a higher rate of distant metastasis, especially to the brain.
Cox’s15 failure-pattern analyses of patients with inoperable or unresectable carcinoma of the lung showed that the extent of the intrathoracic tumor is a major determinant of survival after palliative irradiation or single-agent chemotherapy, especially in patients with squamous cell carcinoma. Saunders and associates58 studied the causes of death in patients with unresectable NSCLC who were treated with a few large fractions of radiation to the modest total dose of 35 Gy. These investigators reported that 72% of their patients died of complications of the intrathoracic disease and only 15% died of distant metastasis. Arriagada and coworkers5 analyzed the effects of local failure relative to distant metastasis in a large prospective trial of induction chemotherapy followed by radiation therapy. The local failure rate, 92% at 5 years, was the reason for the small survival benefit of induction chemotherapy despite highly significant reductions in the distant metastasis rate. Saunders and colleagues57 also demonstrated reductions in the incidence of distant metastasis from improved local control through the use of continuous hyperfractionated accelerated radiation therapy (CHART).
Evaluation before Treatment
The evaluation of patients with carcinoma of the lung apparently confined to the thorax differs little regardless of whether the treatment is to be surgical resection or radiation therapy and chemotherapy. The intent of the pretreatment evaluation is to determine the extent of the local and regional manifestations of the intrathoracic tumor and investigate the most likely sites of distant metastasis.
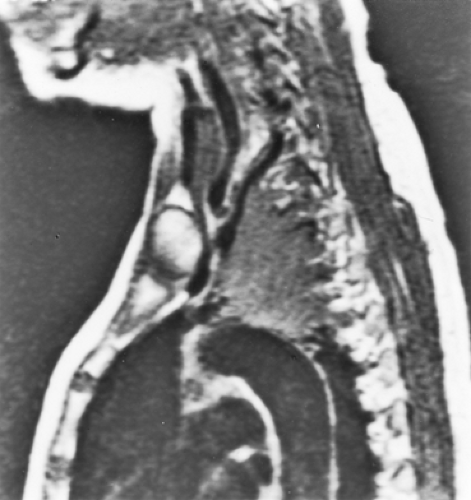 Figure 115-1. MRI reconstruction in the parasagittal plane showing superior extensions of adenocarcinoma of the left apical sulcus. |
A complete blood count, biochemical survey, and posteroanterior and lateral chest radiographs are routine components of the evaluation. The intrathoracic tumor can be assessed well by high-resolution computed tomography (HRCT) with intravenous contrast administration. Magnetic resonance imaging (MRI) has not been found to be more sensitive or accurate than CT by Webb and colleagues,77 although apical sulcus (Pancoast’s) tumors are well demonstrated by MRI, especially in parasagittal planes (Fig. 115-1). As reported by Pieterman and coinvestigators,54 FDG-PET can reveal the intrathoracic and extrathoracic spread of disease. FDG-PET has become the current standard of pretreatment imaging owing to its ability to reveal both intrathoracic extent of disease and extrathoracic metastasis with a single study.
The diagnosis of adenocarcinoma, large-cell carcinoma, or small-cell carcinoma justifies contrast-enhanced CT or MRI of the head to identify occult cerebral metastasis. In patients with no neurologic symptoms, the frequency of occult cerebral metastasis recorded by Jacobs34 and Tarver68 and their associates ranged from 10% to 20%. The finding of cerebral metastasis, of course, profoundly affects the prognosis as well as the plan of therapy.
Radiation Therapy As A Surgical Adjuvant
Radiation therapy is beneficial for a subset of patients with resectable carcinomas of the lung. However, radiation therapy can have an adverse effect on pulmonary function among patients who have undergone major resections of the lung, so only those at high risk for regional recurrence should be treated. Preoperative or postoperative radiation therapy has no place in the treatment of patients without surgical or pathologic evidence of metastasis to mediastinal lymph nodes.
The excellent review by Van Houtte and Henry73 summarized trials of both preoperative and postoperative radiation therapy. Those authors concluded that preoperative irradiation is of no benefit for patients with clearly operable tumors; on the other hand, its possible benefit for patients with marginally resectable tumors, especially those with spread to mediastinal lymph nodes, continues to be studied. Chemotherapy given concurrently with radiation therapy followed by thoracotomy and resection, as reported by Albain,3 is being compared with chemotherapy and radiation therapy without surgery in a prospective intergroup trial.
A meta-analysis of clinical trials of postoperative irradiation by the PORT Meta-analysis Trialists Group1 found that it was of no value for patients with hilar lymph node involvement. The data were insufficient to draw conclusions concerning patients with mediastinal lymph node metastasis in this study. However, a study based on the Surveillance, Epidemiology, and End Results (SEER) database found a survival advantage with postoperative irradiation of patients with mediastinal lymph node involvement (N2).41 It is standard practice for us to recommend postoperative radiation therapy with three-dimensional conformal radiation therapy or intensity-modulated radiation therapy. It is possible to limit the volume of normal lung irradiated such that pulmonary complications are reduced.
Radiation in Definitive Therapy for Squamous Cell Carcinoma, Adenocarcinoma, and Large-Cell Carcinoma
Radiation therapy is an essential component of treatment with curative intent for patients with inoperable NSCLC. Once the determination has been made that the disease is unresectable and a thorough evaluation including CT and PET scanning has shown no evidence of distant metastasis, technically sophisticated, high-dose radiation therapy offers the best opportunity for long-term disease-free survival. In patients with few symptoms (good performance status) and a weight loss of less than 5%, chemotherapy should be combined with irradiation. As Arriagada and colleagues4 have pointed out, standard radiation therapy is far from sufficient local treatment. Moreover, even effective thoracic irradiation does not contribute to the control of already established subclinical extrathoracic metastases. The evolution of more effective systemic therapy reinforces the importance of locoregional control: The actual cause of death is more often the result of intrathoracic complications of the tumor rather than distant organ involvement.
Determination of the Treatment Volume
Definitive irradiation for lung cancer is predicated on irradiation of the primary tumor and involved lymph nodes with adequate margins. Whereas ipsilateral and contralateral hilar and mediastinal lymph node regions and ipsilateral supraclavicular lymph node regions were treated in the past, PET-CT scanning now permits irradiation of only PET-positive disease or enlarged lymph nodes.
Treatment Planning
Treatment planning to achieve permanent control of intrathoracic tumors has greatly increased in sophistication and complexity. This area of intensive research and rapid change is described in detail in Chapter 109; a brief description follows here.
Image-guided radiation therapy11 has the goal of increasing the dose to the tumor while sparing the normal tissues. A PET-CT scan obtained for staging purposes can be used for delineation of the gross tumor volume (GTV) in each axial slice as well as delineation of critical normal structures (i.e., both lungs, spinal cord, heart, and esophagus).2 Tumor motion must be taken into account with thoracic tumors. Such motion during the respiratory cycle can be assessed using 4D-CT.47 Coregistration of FDG-PET images and the 4D planning CT scan offers improved target coverage. Multiple fields may be used with individualized external blocks or machine-based collimation, which encompass the tumor but exclude all normal tissue possible.
The next step in the planning process is to expand the GTV to include sites of suspected subclinical disease. The resulting clinical target volume (CTV) often includes the ipsilateral mediastinum and hilum. Given that parenchymal lung tumors can move with respiration by as much as 1 or 2 cm, as documented by Shimizu and collaborators,65 studies of tumor motion have led to definition of another concept, internal target volume (ITV).2 Treatment planning and treatment with respiratory gating or expansion of the GTV to include the ITV take tumor motion into account, as noted by Vedam and colleagues.75 Yet another variable, the planning target volume (PTV), is an expansion of the CTV to account for day-to-day variations in setup, including tumor motion. The use of PET–CT, as suggested by Vanuytsel and associates,74 to define the GTV, especially that which includes lymph nodes, can significantly reduce the PTV and spare the normal lung. These subjects are covered in depth elsewhere.11
Conformal radiation therapy may also permit use of higher doses of cytotoxic chemotherapeutic agents. Fossella and coworkers25 found it possible to increase the dose of gemcitabine given concurrently with irradiation to a greater extent when 3D conformal radiation therapy was used to exclude more of the esophagus than was possible with 2D treatment planning.
Sophisticated treatment planning is now considered standard. At least 3D conformal radiation therapy planning and delivery or intensity-modulated radiation therapy must be used to limit doses to normal tissues and increase doses in tumors.
Dose–Time Relations
The interplay among the individual dose of irradiation (fraction size), the frequency at which that individual dose or fraction is delivered, the total dose to the tumor and to normal tissues, and the overall time of treatment is encompassed by the term dose–time relations or fractionation.
The Radiation Therapy Oncology Group (RTOG) conducted an important multicenter, centrally randomized prospective trial to compare the effectiveness of 40, 50, and 60 Gy, delivered in 2.0-Gy fractions at five fractions per week for 4, 5, or 6 weeks, respectively. Analyses of this trial by Perez and associates52 revealed a dose–response relation for tumor control in which higher total doses led to lower failure rates. Long-term follow-up of the patients in this study showed no difference in median survival time but did show a highly significant direct relation between 2- and 3-year survival rates and total dose. A total dose of at least 60 Gy in 30 fractions in 6 weeks subsequently became a standard for RTOG studies and for many institutions in the United States.
This fractionation schedule was also used after induction chemotherapy by Dillman23 and Sause61 and their coinvestigators, but Arriagada and associates4 demonstrated that rates of tumor persistence or recurrence were very high after such treatment.
Hyperfractionation with higher total doses of radiation did not improve survival, as noted by Schaake-Koning and colleagues,62 but accelerating treatment by using CHART did, according to Sause.60 Graham30 and Hayman32 and their associates found that conformal irradiation permits higher total radiation doses to be delivered, but the benefit of higher doses in terms of survival has yet to be demonstrated. Considerable interest has been expressed in using conformal techniques to increase fraction size as a means of improving tumor control. Shimizu and associates65 from Hokkaido have used highly conformal irradiation to deliver very large fractions to treat small lung tumors.
As one of us (J.D.C.)16 observed, large-dose fractions have been used for treatment schemes involving hypofractionation (<5 treatments per week), rapid fractionation (large doses 5 days per week for only 3 or 4 weeks), and split-course radiation therapy in which fractions larger than 2.0 Gy are used in an
attempt to compensate for a treatment interruption lasting 1 week or more. A large body of data from radiation therapy for common epithelial tumors at many sites suggests that use of fractions >2.0 Gy and treatments less frequent than 5 days per week are disadvantageous. In reviewing RTOG studies conducted over a 15-year period, Perez51 reported significantly higher rates of late reactions in normal tissues when fractions larger than 2 Gy were used. These adverse consequences of large-dose fractionation were seen in the 2D era. With conformal techniques, small tumors can be controlled consistently with hypofractionation.44
attempt to compensate for a treatment interruption lasting 1 week or more. A large body of data from radiation therapy for common epithelial tumors at many sites suggests that use of fractions >2.0 Gy and treatments less frequent than 5 days per week are disadvantageous. In reviewing RTOG studies conducted over a 15-year period, Perez51 reported significantly higher rates of late reactions in normal tissues when fractions larger than 2 Gy were used. These adverse consequences of large-dose fractionation were seen in the 2D era. With conformal techniques, small tumors can be controlled consistently with hypofractionation.44
Proliferation of Tumor Cells During Treatment
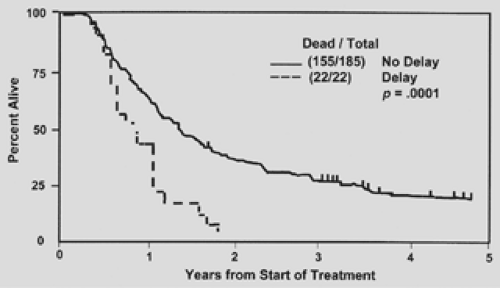
An expanding body of evidence suggests that failure to control common epithelial tumors results in part from continued proliferation of surviving tumor cells during treatment. Fowler and Lindstrom26 reviewed 12 sets of data on radiation therapy for carcinomas of the upper respiratory and digestive tracts and concluded that interruptions of treatment reduced tumor control by approximately 14% for every week of prolongation. The adverse consequences of prolonging treatment time in NSCLC have been shown by one of us (J.D.C.) and associates18 from the RTOG (Fig. 115-2).
Several means of overcoming the proliferation of clonogenic cells during treatment are being investigated. Diener-West and colleagues22 noted that hyperfractionation (i.e., giving larger numbers of smaller-than-standard fractions of irradiation) permits higher total doses to be delivered in the same overall treatment time. An RTOG report by one of us (J.D.C.) and colleagues17 showed a higher survival rate among patients given 69.6 Gy in twice-daily 1.2-Gy fractions than among those given lower total doses. These findings led to a randomized comparison of hyperfractionation with 69.6 Gy given in twice-daily 1.2-Gy fractions and 60 Gy given in once-daily 2.0-Gy fractions (RTOG 88–08/Eastern Cooperative Oncology Group [ECOG] 4588). Sause and colleagues61 initially reported that the hyperfractionation scheme produced no improvement, but at longer follow-up one of us (R.K.) and associates35 showed that 3-year survival rates were higher in the hyperfractionated irradiation group. Lee and associates43 combined that fractionation schedule with concurrent cisplatin and oral etoposide and found a 5-year survival rate of 22% among a small group of patients with inoperable NSCLC.
Accelerated fractionation schemes seek to short-circuit proliferation of residual tumor cells by completing the total course of treatment in the shortest time. Such schemes typically involve delivering more than one fraction per day for all or part of the therapeutic regimen; each fraction is nearly the same dose as that used in a once-daily treatment plan, and the total dose is similar to that achieved with standard fractionation. The most accelerated regimen used to date was studied at the Mount Vernon Hospital in the United Kingdom by Saunders and Dische and associates.57,58,59 In that CHART regimen, 1.5 Gy was delivered three times daily, with 6-hour interfraction intervals and no interruption for the weekend, to achieve a total dose of 54 Gy in 36 fractions in 12 days. Saunders and colleagues57 reported a significant improvement in survival among patients given CHART versus those given standard fractionation therapy. Because most of the patients in that study had squamous cell carcinoma, the relevance of CHART for patients with adenocarcinoma or large-cell carcinoma remains to be determined.
Use of concurrent chemotherapy and radiation therapy is another means of accelerating treatment. Schaake-Koning and colleagues62 found that the addition of cisplatin, administered weekly or daily during radiation therapy for inoperable NSCLC, produced significant improvements in locoregional tumor control (Fig. 115-3A) and survival (Fig. 115-3B). In general, induction chemotherapy followed by standard radiation therapy has not improved local control rates, although Arriagada and colleagues4 observed an apparent reduction of distant metastasis. The potential of increasing local control and improving survival for patients with unresectable NSCLC by using concurrent combination chemotherapy and radiation therapy has been confirmed (see below).
Results of Radiation Therapy for Inoperable Non-Small-Cell Carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree