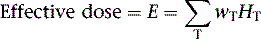Chapter 10 Radiation Considerations for Cardiac Nuclear and Computed Tomography Imaging
INTRODUCTION
The contribution of nuclear cardiology and computed tomography (CT) to the diagnosis and management of cardiovascular disease is undeniable. In contrast to other imaging modalities, they rely on the use of ionizing radiation, which has been associated with the risk of harmful effects. Of greatest concern is increasing the risk of cancer occurrence and mortality.1–4 In recent years, the number of diagnostic imaging studies using “low-level” doses of ionizing radiation increased dramatically. Concern over the risk to the population grew accordingly.2,3 Industry data show that cardiac imaging applications using single-photon emission computed tomography (SPECT), positron emission tomography (PET), and CT experienced greater relative increases compared with other imaging areas. Within these groups, there was an increase in the proportion of studies performed in women and pediatric populations, raising additional concern over exposure in these populations.3,4 These developments have fueled the debate over the accuracy and appropriateness of the estimated rates of cancer predicted by current models and assumptions that have been at times highly disputed. While it is generally accepted that excessive exposure to ionizing radiation can cause certain types of malignancies and other diseases, this relationship is far less accepted for the amounts of radiation used in most diagnostic imaging tests. In clinical practice, the debate is largely centered on the uncertainty in risk estimates relative to the counterprevailing risk of misdiagnosed disease or underutilization of appropriate testing.5 In parallel, advances in imaging technology have reduced radiation dose while preserving image quality, particularly for CT—and cardiac CT specifically—and are rapidly being adopted into clinical application.6 New SPECT reconstruction algorithms and detector configurations for myocardial perfusion imaging allow studies to be acquired in half the time or less7–9 of conventional protocols and theoretically may allow tradeoff of acquisition time advantage for imaging with less injection activity.7 Cardiac PET studies overall deliver favorable radiation dose compared with SPECT and are therefore an option for radiation dose reduction.
RADIATION DOSIMETRY
Fundamentals, Definitions, and Quantities
The amount of energy absorbed from incident radiation per unit mass of tissue is defined as the absorbed dose.10 It is expressed in units of rads, where 1 rad is defined as the absorption of 100 ergs per gram of tissue (or 1 joule per kilogram [J/kg]). The absorbed dose definition applies to most forms of radiation relevant to medical imaging, including photons, (x-rays, gamma) and particles with mass (e.g., electrons, positrons). The International System (SI) unit for absorbed dose is the gray (Gy); 1 gray is equal to 100 rads, or 1 rad = 10 mGy. Absorbed dose is also expressed in terms of the roentgen (R), a unit of radiation exposure defined as the amount of radiation producing 2.58 × 10−4 coulombs (C) of ionization charge per kilogram of air under standard conditions.11 Because this is such a complex definition to relate to various materials, the rad or gray are most frequently utilized.
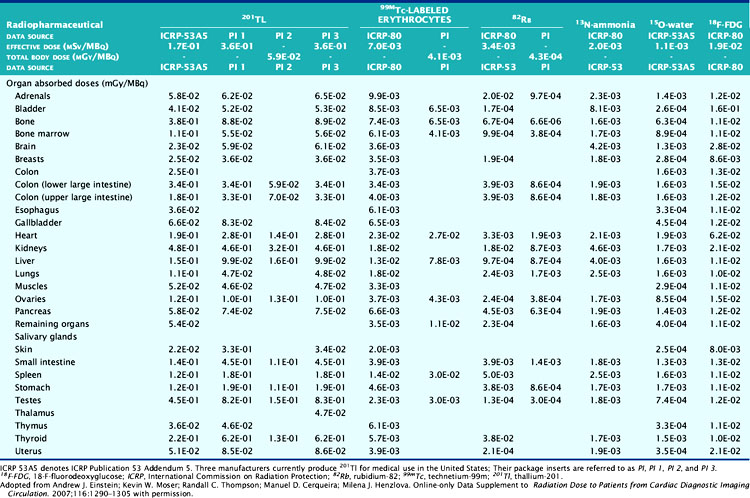
The deposition of energy in tissue occurs through a number of mechanisms that are dependent on the magnitude of energy and atomic properties of the tissue.10,11 Since the same amount of energy deposited by different forms of radiation can produce different amounts of biological damage, a quantity called the quality factor is used to describe the difference.12 The International Commission on Radiation Protection (ICRP), an important policy-setting body for radiation dosimetry, defines the quality factors as: 20 for alpha particles (helium nucleus), 10 for protons, and 1 for beta particles, gamma rays, and x-rays.12 Quality factors are dimensionless quantities defined such that the product of the absorbed dose and quality factor give an estimate of the biological damage for the type and amount of radiation. The resulting quantity is called the dose equivalent and is expressed in units of rem (roentgen equivalent man) or the SI units of sieverts (Sv).10 In principle, dose equivalent and absorbed dose have the same units, since the quality factors are dimensionless. However, to differentiate them, the units of dose equivalent are given the names rems or sieverts. The risk of cancer or harmful effects developing from the biological damage from ionizing radiation are derived from organ-specific dose equivalent values by use of a tissue weighting factor, wT, reflecting the sensitivity to developing fatal cancer, or, in the latest 2007 revision, including lethality and loss of life quality (ICRP-103). Two quantities commonly used to quantify risk are the effective dose (ED)12 and the effective dose equivalent (EDE).13 The ED has generally replaced the older concept of EDE. Recent changes in the wT values in 2007 are an increase for breast tissue (from 0.05 to 0.12), a decrease for gonads (from 0.2 to 0.08), and inclusion of more organs and tissues in the “remainder” component (from 0.05 to 0.12). Table 10-1 lists tissue weighting factors for representative organs from the ICRP-30, ICRP-60, and most recent ICRP-103 publications.13,14 The basic equation relating ED to these quantities is given as:
Table 10-1 Tissue Radiation Sensitivity Factors (wT) from the ICRP-36, ICRP-60, and the Recent ICRP-103 Reports*
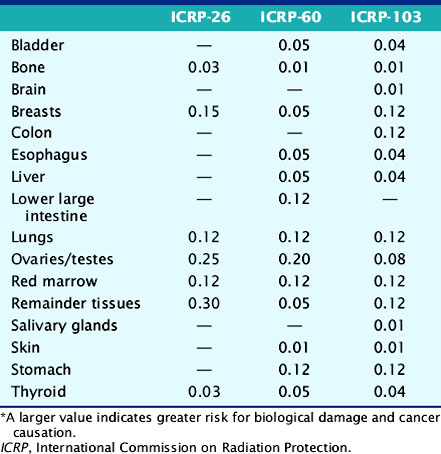
where HT is the tissue-specific equivalent doses and wT is the tissue-specific weighting factors. Complex mathematic simulation programs are used with standardized anatomic models of the human body,15,16 kinetic parameters, and voiding assumptions to estimate HT. Initially in early ICRP models, organs were simulated as spheres containing uniform amounts of radioactivity, and only self-irradiation was included. Two systems exist for calculation of ED and EDE values: the Medical Internal Radiation Dose (MIRD) system,16 developed by the Society of Nuclear Medicine specifically for calculating patient radiation doses, and the ICRP system, developed initially for studying and regulating the protection of nuclear industry workers.13 The MIRD approach includes so-called S-factor’s17 which relate the cumulative activity16 (integral number of decays in an organ) to the exposure of other organs in order to calculate the dose equivalent. A limiting assumption of the MIRD approach is that the radioactivity in each organ is uniformly distributed. Organs containing no activity may have a non-zero absorbed dose value resulting from the surrounding sources. A total body radiation dose is then calculated from the contributions of all organs and compartments (blood, other structures) of the body by a weighted summation of the values that include the “risk” from each organ’s exposure resulting in an equivalent risk from a whole body uniform exposure. Note that the ED is not the same as a “total body dose” often reported on the package insert for many radiopharmaceuticals. The total body dose is the total energy deposited anywhere in the body divided by the total mass of the body. The intent is to provide ED and EDE values that quantify the risk in a single number so that exposures under different circumstances (internal, external, amount of radiation, type of radiation, rate and route of delivery, and other factors) can be compared as a common quantity. The values are intended to be independent of the number of exposures or whether a single or multiple organs were exposed.
Values reported for ED or EDE for a specific application and protocol often differ, leading to uncertainty in evaluating risk.18 This is attributable to differences in assumptions and methodologies in the models and differences in the input data origin. It is important to note that ED and EDE represent expected or average values for a given population. Application to an individual is inappropriate. While radiation dose estimates are provided for an “individual” study by modern CT scanners, it has yet to be implemented for nuclear imaging procedures, as is done for radiation therapy protocols using internal sources.
The package insert (PI) for a radiopharmaceutical is the primary resource for radiation dosimetry values. The dose values are indication-specific and expressed in either rads (rem) or grays (Sv) for a total amount of injected radiopharmaceutical, or “per unit” of injected radiopharmaceutical. The values are typically obtained from studies on normal volunteers under resting conditions, but stress values are included for stress/rest imaging protocols such as myocardial perfusion. The standard stress modality for the PI is exercise stress when given. However, values for pharmacologic stress are often published but are not routine, probably given the many types of pharmacologic stress agents and protocols. The PI also contains relevant dosimetry and safety information for both toxicity and radiation dose contributions from radionuclide contaminants resulting from the radioisotope or radiopharmaceutical manufacturing process.19
The use of ED and EDE as standards for risk is controversial but is the most accepted standard. Individual investigators and position statements reflecting the opinion of health-related professional societies20–22 have questioned the use of this approach. Issues cited in the debate include the omission of molecular repair mechanisms for biological damage in the risk calculations, differences in the tissue radiosensitivity values across species, or differences in the model and specific assumptions in the methodology. Many of the complex factors affecting the development and response by the body to the many forms of cancer are not well understood nor included in the models. The risk values are thus theoretical calculations with limited outcome of data. Adaptation of the EDE and ED is not universal, and some countries have not accepted them as a standard.
Environmental Sources of Radiation Exposure
When comparing risk values for ionizing radiation exposure, it is useful to contrast them with risk values resulting from environmental sources alone. The risk to the general population from background radiation of all types (naturally occurring and man-made) in the United States is estimated at 3.0 to 3.6 mSv annually.23,24 The worldwide annual mean value has been estimated at 2.4 mSv, considerably lower than the U.S. value, although some regions are considerably higher, with a range of 1.5 to 10 mSv.24 One-half to two-thirds of the background radiation dose in the United States is estimated to originate from the alpha particle emissions of radon absorbed by the lungs.23 Individuals living at higher elevations, where shielding by the atmosphere from high-energy cosmic rays is reduced may receive greater exposure. The background values can be helpful when responding to radiation dose questions.
Radiation Dosimetry of Single-Photon Emission Computed Tomography Myocardial Perfusion Tracers
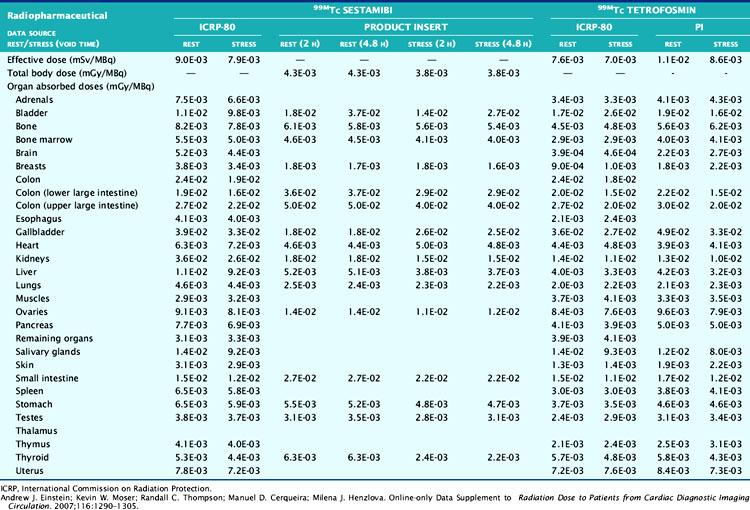
The technetium (Tc)-99m-labeled tracers (T1/2 = 6.02 hours) for myocardial perfusion imaging are Tc-99m sestamibi,25 Tc-99m tetrofosmin26 and Tc-99m-teboroxime,27 although the latter is not currently marketed. Thallium (Tl)-201 chloride (T1/2 = 72 hours) is used for myocardial perfusion and viability assessment in single- and dual-isotope protocols.28 Table 10-2 lists dosimetry values for these tracers from PIs and other sources. Tl-201 dosimetry has attracted particular interest recently because of its high ED and EDE values compared to the Tc-99m perfusion agents and the PET myocardial perfusion agents.3,18 Shown in Table 10-3 are values for Tl-201 SPECT protocols that can be more than double the values for the Tc-99m agents. Using the PI values, Tc-99m tetrofosmin shows an EDE value of 8.61E-03 mSv/MBq for exercise stress and 1.12E-02 mSv/MBq for the resting dose.18 Therefore, a conventional rest/stress SPECT imaging protocol using a 30 mCi (1110 MBq) stress injection and 10 mCi (370 MBq) rest injection yields a total EDE of approximately 14.0 mSv. A rest/stress protocol using Tc-99m sestamibi from the PI values for the same injected activity and protocol yields an absorbed radiation dose of 570 mrad.25 Based on the PI for Tl-201, a stress-redistribution protocol utilizing 3.0 mCi of activity results has an EDE of 37 mSv.18 A dual-isotope SPECT protocol using a 3.0 mCi dose of Tl-201 at rest and a 30 mCi dose of a Tc-99m agent at stress results in an EDE of approximately 41 mSv.3,18 Thus, the inclusion of a Tl-201 injection results in a significant increase in radiation dose compared with the other protocols. Thomas et al. recently examined the dose from Tl-201 chloride to the testes in adults and children, important for fertility considerations. The study recommended a downward revision from the current ICRP values for testicular dose by a factor of almost 2.29 The impact, however, on total ED or EDE values was not significant. For breastfeeding women who must have a nuclear medicine study, a temporary suspension of breastfeeding following the scans currently recommended. The delay (2 weeks) is somewhat unique for Tl-201 studies, given the longer physical half-life. The Nuclear Regulatory Commission (NRC)30 provides specific guidance for the recommended suspension of breastfeeding following a nuclear study, based on the radiopharmaceutical and protocol. Data suggesting that some studies may not warrant suspension of breastfeeding are provided in Refs. 31 and 32. Lastly, radiation dose considerations specific to males and females may be important if the same injected activity is given to both genders, due to organ mass differences, biokinetics, and other factors.32
Table 10-2 ICRP and Manufacturers’ Data on Effective Doses, Total Body Doses, and Absorbed Doses per Unit Activity for Technetium-99m-Tetrofosmin and Technetium-99m Sestamibi Single-Photon Emission Computed Tomography
Radiation Dosimetry for Cardiac Positron Emission Tomography Tracers
Table 10-3 lists radiation dose values for the approved cardiac PET tracers and other cardiac imaging tracers. Rubidium-82 chloride (Rb-82) (T1/2 = 75 sec) is a chemical analog of Tl-201 chloride used for the assessment of coronary artery disease with PET.19,33 Rb-82 is produced as the daughter radionuclide of strontium-82 (Sr-82) eluted from a portable on-site generator renewed approximately monthly due to the physical half-life of Sr-82 (T1/2 = 25 days).19,34 Assay of the eluent for trace contaminants of Strontium (Sr-85) (T1/2 = 64.8 days) and Sr-82 is required as part of daily quality control. SR-82 activity levels toward the end of the month result in a lower maximum available Rb-82 dose for injection, with a concordant reduced exposure.
The Rb-82 PI reports dosimetry values based on data from Kearfott et al.35 in animals and Ryan et al.36 in normal human volunteers. For an adult, an absorbed radiation dose of 0.95 mGy/2220 MBq or 0.096 rads for each 60 mCi injected is reported. Lodge et al.37 computed effective dose equivalent values of 5.5 mSv for combined rest/stress doses of 60 mCi each. Effective dose equivalent values were reported in ICRP-80 of 15.8 mSv for combined rest/stress (60 mCi total) injections.38 Recently, deKemp et al.39 reexamined ED values for Rb-82 myocardial perfusion PET, specifically the role of conservative assumptions used in early dose studies. The authors suggest that the ICRP-80 values may overestimate ED by as much as fivefold, lending weight to the PI values.
Nitrogen-13 ammonia chloride (N-13; T1/2 = 10 min) is used for PET imaging in the diagnosis of coronary artery disease40 and for quantitative assessment of myocardial bloodflow.41 N-13 production requires on-site cyclotron and radiochemistry facilities for rapid preparation and injection into the patient. By comparison, the positrons emitted from N-13 are significantly lower in energy (492 keV) compared to those from Rb-82 and contribute to less radiation dose from N-13 (Valentin et al.) in ICRP-80, reported values for N-13 ammonia chloride for rest/stress injections of 550 MBq (15 mCi) each, equal to 2.2 mSv.41
F-18-labeled fluorodeoxyglucose (F-18DG; T1/2 = 110 min) is utilized with PET cardiac imaging for the assessment of myocardial viability, evaluating the extent of scar, hibernating myocardium, and stunned myocardium.42 FDG provides complimentary information to poorly perfused or equivocal regions on rest/stress images. FDG cardiac imaging is performed conventionally at rest after metabolically preparing the myocardium for optimal FDG uptake. The FDG PI reports dose values for a single 10 mCi injection of FDG.43 ICRP-80 reports that for this amount of activity, the effective dose is 7 mSv.44 The Oak Ridge Associated Laboratories reported an effective dose equivalent 3.0E-02 mSv/MBq, which for a 10 mCi (370 MBq) injection yields 11.2 mSv.44 FDG is provided in unit dose syringes, and thus as with N-13 ammonia, there are important radiation safety considerations for the technologist and clinical personnel involved with the study.45,46 Shown for reference in Table 10-4 are representative values of doses from other common medical imaging procedures using ionizing radiation.
Table 10-4 Radiation Exposure Estimates from Common Medical Imaging Procedures
| Study Type | Relevant Organ | Relevant Organ Dose (mGy or mSv) |
|---|---|---|
| Dental radiography | Brain | 0.005 |
| Posterior-anterior chest radiography | Lung | 0.01 |
| Lateral chest radiography | Lung | 0.15 |
| Screening mammography | Breast | 3 |
| Adult abdominal CT | Stomach | 10 |
| Barium enema | Colon | 15 |
| Neonatal abdominal CT | Stomach | 20 |
CT, Computed tomography.
Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;357:2277-2284.