Fig. 3.1
The three components of a continuous quality improvement program
Quality assurance (QA) – documents outcomes and accuracy as well as assessing adherence to policies, procedures, and other standards. Examples of QA include comparison of noninvasive examination findings to other diagnostic tests or procedures and peer review.
Quality control (QC) – the calibration and maintenance of equipment. This is generally achieved through following manufacturer recommendations for routine maintenance, electrical checks, and software updates. Any QC must be documented and accessible in the event equipment performance is called into question.
Continuous quality improvement (CQI) – the process of becoming a more successful laboratory overall by building upon traditional quality assurance methods and emphasizing the organization and systems.
How Is a Quality Improvement Program Developed?
Developing the laboratory’s quality improvement (QI) plan is probably the easiest part of a successful program. Following a few simple steps prior to implementation will help to avoid possible loopholes and frustrations in the future (Fig. 3.2).
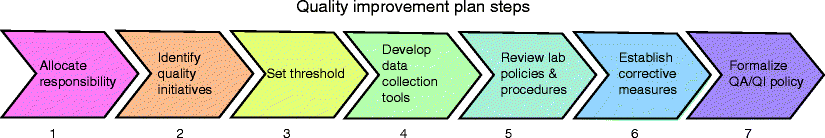

Fig. 3.2
Seven steps involved developing a quality improvement plan
Allocate Responsibility
When developing and implementing a quality improvement program, it is very important to identify key staff to share in the responsibility of supervising and maintaining it. To ensure the success of the program, all levels of management must truly support the initiative and be willing to provide ample time and resources to carry out the duties associated with sustaining an ongoing plan. Integrating the QI responsibility as part of one or more staff members’ written job descriptions helps to clearly define the role of the individual and provides a level of accountability. Depending upon its size and the volume of testing performed in the laboratory, it may be very helpful to establish a QI/QA committee to assist in developing the policies and procedures associated with implementation and continued monitoring of the program. Having multiple people involved in the program helps to provide continuity in the event a staff member holding a key role leaves the establishment.
Identify Quality Initiatives
When thinking of QA from a laboratory’s perspective, it is often limited to the correlation of noninvasive examination results to other diagnostic tests or procedures. Test validation and quality control must be included in the quality improvement program; however, there are additional ways to assess the overall functioning and reliability of the laboratory. Evaluating other areas within the laboratory such as patient satisfaction, appropriateness of testing, safety, report content, report turnaround time, and adherence to testing protocols further strengthens the quality program. The options that may be monitored in the laboratory are endless and integral in enhancing overall laboratory quality.
Set Thresholds
After determining what quality initiatives will be monitored, the laboratory must establish thresholds that will be met for each item. A threshold is essentially an agreed upon percentage of time that positive or negative outcomes are acceptable. For instance, how often is it satisfactory that examination results do not correlate with another diagnostic procedure or patient outcome? Generally the thought is that it is never acceptable; however, there are very few diagnostic tests in medicine that are correct 100% of the time. Thresholds should be achievable, yet not diluted to the point that they are of no value in identifying problems and inconsistencies. When setting thresholds, it is important to take into consideration current industry standards and expectations. Because of the potential differences between laboratories, it is difficult to set firm universal thresholds, and, consequently, there are no standardized thresholds at this time. Meeting positive thresholds of 80% or greater is generally accepted and may be modified after data has been collected over a period of time. Thresholds are set as a baseline for improvement and should be assessed and adjusted. When success rates fall below a minimum or continually exceed expectations, a departmental review should be performed to determine the causes and implement changes to procedures or adjustment to the thresholds if necessary [3].
Develop Data Collection Tools
Once you have identified who will gather the data, what will be collected, and set desired outcomes, the next step is to identify data collection methods. Establishing guidelines for how often information is collected, in what format it will be recorded, and how often it is shared with staff members must be established. There are many ways in which the data can be documented; however, the more complicated the process is, the less likely it is to occur (Fig. 3.3). Identifying very specific information and having simple streamlined forms in which to enter the data lead to a more successful quality improvement program.
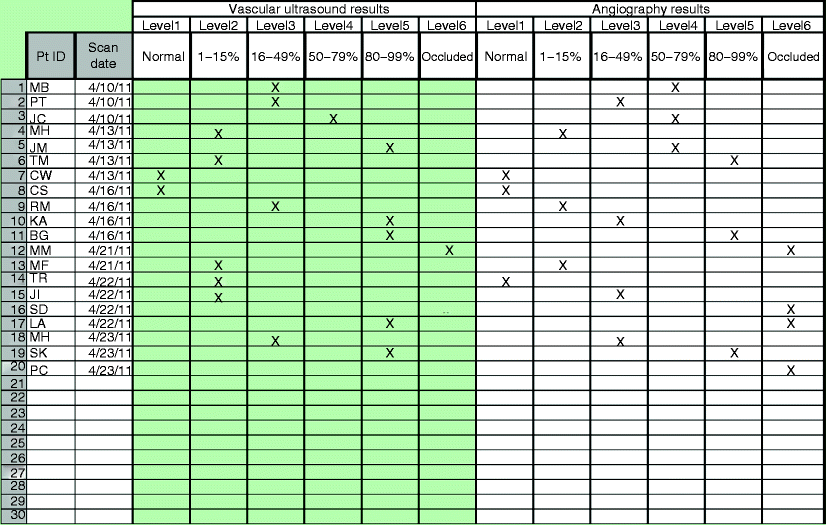

Fig. 3.3
Sample correlation data collection worksheet
Documenting patient examination findings that may be used in obtaining correlation to other tests or procedures may be accomplished through something as simple as a notebook. The notebook can be kept in the testing rooms to catalog patients with positive results that may lead to medical follow-up, another correlative procedure, or potential surgical candidates. As well, there are many software programs that have been developed to assist in tracking QA information. Prior to purchasing additional data collection tools, be diligent in assuring that they meet the needs of your laboratory.
Review Laboratory Policies and Procedures
For the purposes of monitoring examination accuracy and safety, all laboratory policies and procedures should be reviewed annually to assure that they are up to date and include all components necessary for complete documentation and accurate interpretation. Using a checklist in the review of procedures will help to assure all necessary steps are included (Table 3.1). The procedures should be easily accessible to all staff, and steps must be made to guarantee that everyone follows the current procedures.
Table 3.1
Partial example of a checklist that can be used in review of the laboratory’s procedures and protocols
Procedure and protocol review checklist | |||
|---|---|---|---|
Protocol/procedure title: carotid duplex | |||
Date reviewed: April 17, 2011 | |||
Reviewer: C. Weiland | |||
Components of protocol | Present | Absent | Current |
Equipment used | |||
Patient positioning | |||
B-mode image documentation: | |||
RCCA | |||
R bulb | |||
RICA proximal to mid | |||
LCCA | |||
L bulb | |||
LICA proximal to mid | |||
Other abnormal findings | |||
Regardless of the number of sonographers in the laboratory, technical protocols should at a minimum include the following components:
Equipment used
Patient preparation and positioning
Examination techniques
Minimum image/Doppler acquisition
Additional documentation of abnormal and/or incidental findings
Appropriate annotation
In that examinations are performed consistently in accordance with the written protocols, it is as imperative to have standardized diagnostic criteria used in the interpretation of each type of testing performed in the laboratory. Criteria must be applied in the same consistent manner by all interpreting physicians. Most laboratories choose to utilize criteria that have been previously validated and published. If the published criteria are modified to better suit the laboratory processes, equipment, or outcomes, it is important that these amended criteria be internally validated to assure its accurateness.
Routine monitoring of these two vital components of the laboratory processes will help ensure a systematic approach to services rendered by the laboratory and generally result in improved standardization, consistent results, and appropriate patient management.
Establish How Negative Findings Will Be Addressed
If upon analysis of the information collected, weaknesses exist or established thresholds are not being met, there must be a routine method in which problems are addressed. It may be found that deficiencies exist due to procedures or individuals. Regardless of the cause of the inconsistencies or inaccuracies establishing a consistent process of acknowledging problems, establishing a plan for improvement and time frame in which reevaluation will occur is essential.
Later in this chapter we will discuss how using corrective action plans is one method that is useful in identifying and establishing follow-up for virtually any undesirable outcome identified through the QI program.
Formalize the QA/QI Policy
After the previous steps have been completed, a policy and procedure must be written and communicated to all staff members. The policy should include all of the decisions made in steps 1 through 6 so that everyone involved with the laboratory understands the expectations and importance of the quality program.
What Should the Laboratory Assess and How?
In addition to examination validation, included below are areas the laboratory could monitor and how that particular data can be documented and evaluated. Keep in mind the goal is to gain useful information that assists in identifying the strengths and weaknesses within the laboratory as a whole and is useful in making suggestions as to how goals will be achieved.
Patient Satisfaction
It is easy to get caught up in the busy and sometimes impersonal environment that today’s health care breeds. Institutions that have recognized that this pace is negatively impacting the patient’s experience have taken steps to focus on the person. With the many choices patients and primary care providers have when deciding where to receive their diagnostic imaging, assuring a positive patient experience has become paramount in the continued success of the laboratory regardless of its setting. Utilizing simple patient satisfaction surveys will provide valuable information in a simple format (Fig. 3.4). The survey may be provided either at the time of departure, via mail or email after the appointment. By simply documenting, the percentage of patients who measured any part of their experience less than the desired outcome is easily tracked in a spreadsheet and identified problem areas addressed through staff education.




