Pulmonary Tuberculosis and Other Mycobacterial Diseases of the Lung
Gregory Campbell
Jeffrey Glassroth
The mycobacteria are a diverse group of aerobic acid-fast bacilli distributed worldwide in the environment and in animal/human hosts. Many species of mycobacteria have been identified. A number of these are historically important in causing pulmonary disease in humans (Table 90-1), including the Mycobacterium tuberculosis (MTB) complex (M. tuberculosis, M. bovis, and M. africanum), Mycobacterium avium complex (MAC), and Mycobacterium kansasii, to name a few. Disease manifestations in humans are protean, but the lung is the most frequent organ involved. Medically refractory or resistant pulmonary disease is the most common reason for surgical intervention. This chapter discusses the diagnosis, treatment, and other issues related to various mycobacterial diseases and the role of thoracic surgery in their management.
Epidemiology
Mycobacterium tuberculosis (MTB) causes tuberculosis (TB). It is spread, almost exclusively, by the inhalation of infected droplet nuclei produced by patients with pulmonary TB. In fact, only a relatively small percentage (about 10%) of patients infected with MTB will develop clinical (i.e., active) disease. That said, MTB is one of the most common infectious agents affecting humans worldwide. The disease has plagued humanity since antiquity. Only relatively recently has our understanding of the disease evolved sufficiently to allow for the provision of highly effective drug therapy. Indeed, it was more than a half-century from 1882 when Koch identified MTB as the causative organism of TB until effective medical treatment became available. Prior to that time, various forms of collapse therapy and surgical interventions such as thoracoplasty and plombage were extensively applied. With the development of streptomycin in 1946 and isoniazid in 1952, the possibility of durable TB cures—which, in turn, could reduce transmission of disease—became a reality. With that, the use of various forms of surgical interventions began to recede. By the latter half of the twentieth century, the development of multiple other antituberculous drugs, drug combinations, and strategies led to relatively rapid cures for patients with access to medical therapy. Indeed, elimination of TB has become a realistic public health goal, at least in many resource-rich parts of the world.
The attainment of this goal, however, remains elusive, owing in part to the biology of the organism and its relatively slow rate of multiplication, necessitating long periods of treatment. The rising number of cases worldwide since the early 1980s attests to this fact. Additional reasons for this rise include the human immunodeficiency virus (HIV) pandemic (particularly in sub-Saharan Africa), the lack of sufficient resources and infrastructure to combat the infection in high-prevalence areas of the world, the emergence of drug-resistant strains of MTB, and increased immigration from highly endemic areas. It is no surprise that against this background, TB remains one of the most common communicable infectious diseases worldwide, with the nearly one-third of the world’s population who are infected with MTB comprising a pool of several billion infected persons from whom most active cases emerge each year.
Annually, about 9 million new cases and 2 million deaths worldwide are caused by this organism. Twenty-two so-called high-burden countries within sub-Saharan Africa, former European eastern bloc nations, Latin America, and Asia account for about 80% of the world’s new cases each year. Sub-Saharan Africa, where almost one in three patients with TB is coinfected with HIV, has the highest regional incidence and death rate attributable to TB. This reflects HIV coinfection’s effect of “telescoping” the natural history of TB by increasing susceptibility to MTB infection and accelerating progression to active disease once infection occurs. A risk of tuberculous disease of 5% to 15% per year has been estimated for individuals coinfected by MTB and HIV.26 Moreover, inadequate treatment facilitates the emergence of resistant TB, further challenging resource-limited control programs.
In the United States, where a robust public health care infra- structure exists, the burden of MTB is much less and the goal of TB elimination seems more attainable. The lowest incidence of TB since reporting began in 1953, or 4.6 cases per 100,000, was recorded in 2006. However, MTB still poses a significant public health challenge in the United States, and the rate of decline is slowing. This reflects a disproportionate number of cases among foreign-born immigrants and U.S.-born minority populations, with African Americans, Hispanics, and Asians having rates up to 21% greater than those of whites. Within foreign-born and minority populations, the incidence of TB has increased each year since 1993. Multidrug-resistant TB (MDR-TB) is also more common among foreign-born individuals, representing 81% of all U.S. MDR-TB cases in 2005.
Table 90-1 Common and Uncommon Causes of Mycobacterial Lung Disease in the United States | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
In contrast, much less is known about the epidemiology of nontuberculous mycobacteria (NTM), in part because they do not cause reportable diseases for public health purposes. Although infection is very common in some geographic areas, disease due to NTM appears to be much less common28 However, there is a general sense that disease due to some species of NTM may be increasing; in the United States NTM, especially M. avium complex, are now being isolated more commonly than MTB in many laboratories.
Microbiology and Pathogenesis
Mycobacteria are rod-shaped, thin aerobic bacteria measuring approximately 0.5 by 3 μm (Fig. 90-1). Humans are the main reservoir for MTB, although it has been reported in many other animals. Less is known about NTM, sometimes called environmental mycobacteria, because most are presumed to exist in the environment. MTB and many but not all NTM grow slowly, with visible growth apparent by 3 to 6 weeks on solid media. The cell walls of mycobacteria contain high concentrations of mycolic acids and long-chain cross-linked fatty acids, which account for the acid-fast quality of the mycobacteria and whereby the organism cannot be decolorized once treated with Gram’s stain. This cell wall structure confers very low permeability to macromolecules, which in part accounts for the relative ineffectiveness of many conventional antibiotic agents against MTB.
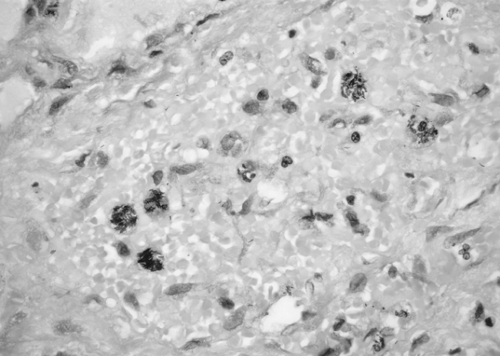 Figure 90-1. Characteristic Mycobacterium tuberculosis acid-fast bacilli (AFB) seen in sputum smear for AFB. Mycobacteria are thin red beaded rods. |
Infection with MTB usually occurs via person-to-person spread of aerosolized or airborne tubercle bacilli from a respiratory tract focus. So-called infected droplet nuclei are produced through coughing—a particularly effective way of aerosolizing MTB—sneezing, or speaking. Extrapulmonary MTB is only rarely contagious because bacilli are typically not aerosolized from those foci. Although it is less certain, NTM inhalation of infected aerosols from environmental sources rather than human-to-human transmission is thought to be the general mechanism of their transmission. Once aerosolized, mycobacteria can remain suspended for hours; once inhaled, the size of the suspended particle helps determine how distal into the respiratory tree the particle is deposited. Smaller particles can bypass the mucociliary ladder and deposit themselves on the surfaces of alveoli, where they are ingested by host alveolar macrophages. From this point forward, and based on our understanding of the pathogenesis of TB, the following seem to be the possible outcomes of an inhalation of mycobacteria: the tubercle bacilli (and presumably NTM) may be destroyed or otherwise contained by host defense mechanisms; the organism may not be destroyed but may multiply and remain dormant (this is termed latent TB infection or LTBI [90% of infections] when MTB is involved); this latent infection may “reactivate” and cause disease at some later time (about 5%–10% of LTBI and a much
smaller proportion of NTM infections). During the weeks immediately following inhalation and infection, the individual is usually asymptomatic. In the case of MTB, lymphohematogenous spread of the organism and the development of specific immunity as manifest by tuberculin skin test reactivity occur. This period of systemic spread from the lungs creates the potential for extrapulmonary as well as pulmonary disease. Uncommonly, MTB may cause clinical tuberculosis immediately, which is then called primary tuberculosis. The lifetime risk of tuberculosis (i.e., disease due to MTB) developing is about 10% among immunocompetent persons infected with MTB. A much higher proportion of immunocompromised persons (e.g., those who are HIV-coinfected) will likely develop active tuberculosis.
smaller proportion of NTM infections). During the weeks immediately following inhalation and infection, the individual is usually asymptomatic. In the case of MTB, lymphohematogenous spread of the organism and the development of specific immunity as manifest by tuberculin skin test reactivity occur. This period of systemic spread from the lungs creates the potential for extrapulmonary as well as pulmonary disease. Uncommonly, MTB may cause clinical tuberculosis immediately, which is then called primary tuberculosis. The lifetime risk of tuberculosis (i.e., disease due to MTB) developing is about 10% among immunocompetent persons infected with MTB. A much higher proportion of immunocompromised persons (e.g., those who are HIV-coinfected) will likely develop active tuberculosis.
The progression of MTB infection to TB disease (pulmonary or extrapulmonary) is dependent on factors endogenous to the host and on poorly defined virulence factors of the infecting organism. Host factors include innate (genetic) susceptibility to the disease, the status of cell-mediated immunity (e.g., HIV coinfection), and other factors that increase susceptibility, such as diabetes, silicosis, and chronic renal insufficiency. Gagneux and colleagues36 demonstrated an association between specific worldwide mycobacterial lineages and human populations, suggesting a human–pathogen coevolution for infectivity likely based on histocompatibility. The observation that the histocompatibility DRB1*1501 allele is associated with advanced disease and failure to respond to drug therapy whereas the DRB1*1502 allele is associated with less risk of disease progression underscores the importance of genetic susceptibility in individuals.30 The risk for the development of multidrug-resistant TB may also be histocompatibility “conditioned.”59 Specific mutations in the genes responsible for interferon production or interferon receptors (important for the host response to MTB) result in a higher risk of developing active disease.25,68 Other potential non-HLA host factors contributing to susceptibility to active disease include polymorphisms in transporters associated with antigen processing (TAP), Toll-like receptors, SLC11A1, vitamin D receptors, and mannose-binding proteins, to name a few.6
If MTB infection occurs in a host with a normal immune system, particularly a normal cell-mediated immunity (CMI), the MTB–host interaction progresses through stages.91 Following ingestion of the MTB by the resident alveolar macrophages, the MTB population within the macrophages increases in what is called the logarithmic stage. In this stage, the CMI has not yet been fully activated against the MTB, and often the innate antimycobacterial properties of the macrophages are not sufficient to destroy the organisms. Cell wall components, such as lipoarabinomannan, may be responsible for interrupting presentation pathways that would normally clear the organism.118 Organisms translocate to regional lymph nodes (i.e., lymphohematogenous dissemination) and spread to other organs of the body. Next is the immunogenic phase, where the CMI has been activated, with helper T cells activating macrophages in the areas of infection while cytotoxic T cells kill the tubercle-filled macrophages as part of the delayed-type hypersensitivity reaction—a so-called Th1–mediated process. This process is responsible for the formation of a microscopic caseous necrotic center of a tuberculous focus. Tumor necrosis factor alpha (TNF-α) is crucial in this process.3,66 The infection at this point may remain contained locally and at distant sites as a self-limited primary infection; the only manifestation of its presence may be the tuberculin skin test reaction, which develops some 8 to 12 weeks following infection in immune-competent hosts. This defines the concept of latent MTB infection—an enclosed, asymptomatic, nontransmissible state in which MTB is either in a noncycling persistence or a balance of growth and death.99 Latency is dependent on a competent CMI.
For reasons that are not completely clear, the host–tubercle bacillus interaction at the center of the granuloma can remain stable or may deteriorate with renewed MTB replication (reactivation) and possibly progress to liquefaction and cavitation. During this phase, endobronchial spread of infection to other lung segments and/or renewed hematogenous dissemination can occur. It is thought that an individual’s immunity, influenced by comorbid factors, determines whether reactivation occurs.
Although less is known about the NTM, it is presumed that similar interactions apply to infections with many of these organisms. While some of the same conditions of immune compromise affecting MTB also increase the risk of NTM disease, other conditions such as cystic fibrosis, gastrointestinal conditions such as achalasia, chronic obstructive pulmonary disease (COPD), and prior pulmonary TB may also increase the risk of disease due to certain species of NTM.35,61,71
Clinical Manifestations of Pulmonary and Related TB
The lungs are the most common site of TB. However, because of the phase of lymphohematogenous dissemination, tuberculosis can involve any organ system in the body. Following the lungs, in descending order of frequency of involvement, are the lymph nodes, pleura, genitourinary tract, and bones and joints. Generalized spread (i.e., miliary TB) and meningitis are even more uncommon forms of disease. Involvement of the gastrointestinal tract and of the adrenal cortex, producing adrenal insufficiency, is also possible.93,121 Pulmonary disease may coexist with extrapulmonary disease.
Pulmonary Disease
Pulmonary disease represents the majority of MTB active disease cases occurring in immunocompetent patients. Clinical syndromes include primary tuberculosis and postprimary tuberculosis. The distinction between these two presentations is based in part on the chest radiograph and history of prior TB infection. Although there is much overlap in these presentations, they are still convenient for the conceptualization of TB. Most adult patients who are not immunocompromised will have the postprimary syndrome, often with a presentation involving the upper lobe or a superior segment of the lower lobe. However, it should be noted that as many as one-third of pulmonary TB patients will present with radiographs that diverge from this classic teaching. Moreover, persons coinfected with HIV can have very atypical radiographs and even clear lung fields when they present with pulmonary TB.38
Primary Pulmonary Tuberculosis
Primary tuberculosis occurs soon after the initial MTB inoculum has entered the local alveolar macrophages. In most individuals, the host immune response contains the infection within a
few weeks, leaving a small granuloma that may calcify over time (Ghon lesion). However, in patients who cannot contain the initial infection because of impaired host CMI, poor nutrition, or age (i.e., young children), the infection may progress. The clinical and radiographic features of primary TB relate to lung parenchymal involvement with intrathoracic lymph node enlargement, including acute hematogenous dissemination with miliary lung involvement, TB pneumonia with or without pleural effusion, and intrathoracic adenopathy with bronchial compression and atelectasis.
few weeks, leaving a small granuloma that may calcify over time (Ghon lesion). However, in patients who cannot contain the initial infection because of impaired host CMI, poor nutrition, or age (i.e., young children), the infection may progress. The clinical and radiographic features of primary TB relate to lung parenchymal involvement with intrathoracic lymph node enlargement, including acute hematogenous dissemination with miliary lung involvement, TB pneumonia with or without pleural effusion, and intrathoracic adenopathy with bronchial compression and atelectasis.
Radiographically, most primary TB involves the mid- to lower lung zones, where ventilation is greatest and inhaled MTB-infected droplet nuclei are most likely to have been deposited. The lung involvement is often segmental or lobar, with consolidation suggestive of more common bacterial pneumonia. The presence of centrilobular nodules and branching linear structures around distal airspaces corresponds pathologically to caseous material filling bronchioles mainly in early active disease.49,50 This pattern is the so-called tree-in-bud pattern. Yet this pattern is not pathognomonic of active TB and can be related to a variety of disorders.94 Pleural effusions, due to pleural extension of the parenchymal process with subsequent immunologic reaction in the pleural space, may be seen, as may hilar or mediastinal lymph node enlargement.65,103
Patients can present with constitutional symptoms as well as purulent cough and dyspnea. Additionally, they can have symptoms attributable to mediastinal or hilar lymphadenopathy, or both, such as wheezing or, particularly in children, acute upper airway obstruction. The latter is seen less frequently in the modern era of effective antimycobacterial therapy. If treatment is necessary for lymph node compression, surgery has been the therapy of choice,78,120 although corticosteroids in children with acute bronchial obstruction has also been employed as an adjuvant to medical therapy of the MTB.105 Intrathoracic lymphadenopathy is not limited to primary TB, however. Up to 5% of 56 patients described in one report of adult postprimary disease had sizable, nonobstructing intrathoracic lymphadenopathy.119 Younger patients may also have cutaneous immunologic phenomena, such as erythema nodosum or erythema induratum.
HIV-infected patients represent an important group of patients who may develop progressive primary TB infection, and may demonstrate atypical patterns of disease. According to Long and associates67—before the era of effective highly active antiretroviral therapy (HAART)—HIV-seropositive patients with prior AIDS-defining events and advanced HIV infection are the most likely to present with a primary TB radiographic pattern followed by HIV-positive patients without AIDS and immunocompetent HIV-seronegative patients with 80%, 30%, and 11% respectively of TB cases in each group having a primary pattern. The variability of radiographic findings correlates with CD4 count with more atypical patterns occurring as CD4 count declines.81 HAART is associated with postprimary pattern as compared with those with evidence of severe immunosuppression not on HAART.12
Postprimary Tuberculosis
Postprimary tuberculosis—also referred to as adult-onset TB, reactivation TB, or secondary TB—results from reactivation of latent TB infection in a variety of at-risk persons (Table 90-2) but can also occur in persons with no identifiable “risk factors.” Any organ system may be the site of reactivation, but the chest is involved in over 80% of immune-competent adults; a much higher proportion of HIV-infected persons will have extrapulmonary disease.121
Table 90-2 Persons at Particular Risk for Reactivation of Latent MTB Infection | |
|---|---|
|
Radiographically, postprimary TB in the chest often presents in the apical or posterior segments of the upper lobes or the superior segments of the lower lobes, where alveolar oxygen tension is the highest. Upper lobe alveolar infiltrates (TB pneumonia), thick-walled cavities that often have smooth inner walls, and, over time, fibrosis with volume loss are some characteristic features of postprimary disease. These features contrast with those of primary TB (Table 90-3). Up to 30% of chest radiographs can have presentations that are considered atypical for postprimary disease (e.g., mediastinal lymphadenopathy, lower lung zone predominance, single or multiple nodules, or isolated pleural effusions).58,63 Indeed, it has become more difficult to discern primary and postprimary disease based on radiographic findings.110 In particular, HIV-positive patients who are severely immunosuppressed (CD4 cell count <0.02/L) are more likely to present with atypical features in the setting of reactivated disease. This includes intrathoracic lymphadenopathy, which on chest computed tomography (CT) may have a low-density quality with contrast enhancement of the periphery.79
Reactivated pulmonary TB can be asymptomatic or can present with persistent cough, constitutional symptoms (fever, malaise, night sweats, and/or weight loss), nonresolving pneumonia with dyspnea and hemoptysis, or any combination of these. Cough is the most common symptom and may be associated with hemoptysis, which is often mild but can be severe, as when associated with erosion of a bronchial artery vessel within the wall of a TB cavity (Rasmussen’s aneurysm). In a meta-analytical review, Perez-Guzman and associates80 found that constitutional symptoms are often a prominent feature of the presentation, particularly in younger patients, whereas dyspnea tends to be a prominent early complaint in the elderly, given the higher prevalence of coexisting cardiopulmonary disease in this population.
Physical exam and laboratory abnormalities may assist in the diagnosis of tuberculosis. Because of the possibility
of extrapulmonary disease, attention to extrapulmonary abnormalities may yield valuable diagnostic information. Some commonly encountered laboratory abnormalities include a normocytic, normochromic anemia, an elevated erythrocyte sedimentation rate, leukocytosis (sometimes with a monocytosis), hyponatremia and sterile pyuria (due to genitourinary tract involvement).
of extrapulmonary disease, attention to extrapulmonary abnormalities may yield valuable diagnostic information. Some commonly encountered laboratory abnormalities include a normocytic, normochromic anemia, an elevated erythrocyte sedimentation rate, leukocytosis (sometimes with a monocytosis), hyponatremia and sterile pyuria (due to genitourinary tract involvement).
Table 90-3 Comparison of Chest Radiographic Features of Primary and Postprimary Tuberculosis in Immunocompetent Persons | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Pleural Tuberculosis
Pleural tuberculosis is a relatively common associated manifestation of pulmonary parenchymal TB, with up to 7% of active disease complicated by related pleural effusions.2 Pleural TB can occur in relation to both primary and postprimary radiographic presentations or can occur in isolation. Postprimary (reactivation) pulmonary TB is the most common associated pattern.74 Patients often present acutely with nonproductive cough and pleuritic chest pain.40
Pleural TB usually involves an initial neutrophilic pleuritis and then over a period of some days, a characteristic lymphocyte-predominant (>80%) exudative effusion that is typically unilateral, small to moderate in size, and rarely compromises the patient’s respiratory status. However, large effusions can sometimes occur. The use of CT or ultrasound may help identify pleural disease.17
Diagnosis
Diagnosis of Infection
The diagnosis of TB infection can be made through the use of targeted tuberculin skin testing (TST) or the more recently developed whole blood interferon-gamma release assays (IGRA). The diagnosis of MTB infection is usually pursued for one of several reasons: as part of an institutional epidemiology program to determine the rate of transmission of MTB; to identify and treat groups of patients with LTBI who are at risk for progressing to disease because infection with MTB is a prerequisite for disease, or to evaluate a patient who may be ill with tuberculosis. The tuberculin skin test is the most commonly used testing method for these two indications.
The tuberculin skin test uses purified protein derivative (PPD) from a culture filtrate of MTB. In the United States, it is injected intradermally as a 5–tuberculin unit (TU) dose on a clean area of skin on the forearm. A delayed-type hypersensitivity reaction will usually occur in the form of induration at the site of injection usually 48 to 72 hours later if the patient has an intact CMI and has been infected and sensitized by MTB or antigenically related mycobacteria. The maximal diameter of the induration (not erythema) is then determined. In general, the larger the reaction size, the more likely that the individual is infected with MTB. The test does not determine presence of or extent of disease (i.e., TB).
When used in screening for LTBI, tuberculin skin test should be given to persons who are at highest risk for progressing to active disease. By adjusting the threshold for “positive” reaction, the sensitivity of the test can be increased (small reaction size lowers threshold) or decreased (larger reaction size elevates threshold). Reciprocal changes in the test specificity can be expected. Interpretation of the results reflects risk stratification and is summarized in Table 90-4. The lowest threshold for a positive test (i.e., greatest sensitivity) is assigned to the highest risk stratum.104 Unfortunately, the TST has both a limited sensitivity and specificity and may fail to identify patients at highest risk.48 In persons with active TB, a positive tuberculin skin test is supportive of the diagnosis, but a negative test does not exclude the diagnosis. Persons with miliary TB, pleural TB, and TB meningitis, as well as advanced HIV-infected and malnourished patients, can all have negative tuberculin skin tests even with active TB.
There are two available interferon assays: the second-generation enzyme-linked immunosorbent assay (ELISA), based QuantiFERON-TB Gold (Cellestis Limited, Victoria, Australia), and the enzyme-linked immunospot (ELISpot) based T-SPOT.TB (Oxford Immunotec, Oxford, UK). Initial guidelines were published regarding the use of the first-generation QuantiFERON-TB test,73 which is no longer available for use, and recently for QuantiFERON-TB Gold.72 T-SPOT.TB has been
approved in Europe and guidelines are available108— it is being reviewed by the U.S. Food and Drug Administration (FDA). Both tests rely on proteins (early secretory antigenic target protein 6, or ESAT-6; culture filtrate protein 10, or CFP10) unique to clinical isolates of MTB but absent from the majority of nontuberculous and BCG strains.4 ESAT-6 and CFP10 are potent targets of T lymphocytes secreting IFN-γ. While these tests have some notable differences, they both seek to overcome the deficiencies of the TST and have shown higher specificity. Recent evidence supports a higher sensitivity/specificity for T-SPOT in latent TB, both in BCG vaccinated individuals and those who are immunocompromised.34,64,84 Yet within the severely immunocompromised HIV population, falsely negative results should still be expected.69 A newer formulation of QuantiFERON-TB Gold, “in-tube,” seeks to improve specificity by adding an additional antigen.9 These assays do not currently perform well in the setting of active disease.52 At present in the United States it is recommended that the new IGRA can be used as alternatives to TST in all settings where TST is currently used to test for LTBI.72 These tests have the advantage of rapid objective diagnosis (<24 hours) and no need for return interpretation. As with any test, false positive and negative results can occur, and results must be considered in relation to risk and clinical setting. Currently, TST should be used to test for MTB infection when active disease is present.
approved in Europe and guidelines are available108— it is being reviewed by the U.S. Food and Drug Administration (FDA). Both tests rely on proteins (early secretory antigenic target protein 6, or ESAT-6; culture filtrate protein 10, or CFP10) unique to clinical isolates of MTB but absent from the majority of nontuberculous and BCG strains.4 ESAT-6 and CFP10 are potent targets of T lymphocytes secreting IFN-γ. While these tests have some notable differences, they both seek to overcome the deficiencies of the TST and have shown higher specificity. Recent evidence supports a higher sensitivity/specificity for T-SPOT in latent TB, both in BCG vaccinated individuals and those who are immunocompromised.34,64,84 Yet within the severely immunocompromised HIV population, falsely negative results should still be expected.69 A newer formulation of QuantiFERON-TB Gold, “in-tube,” seeks to improve specificity by adding an additional antigen.9 These assays do not currently perform well in the setting of active disease.52 At present in the United States it is recommended that the new IGRA can be used as alternatives to TST in all settings where TST is currently used to test for LTBI.72 These tests have the advantage of rapid objective diagnosis (<24 hours) and no need for return interpretation. As with any test, false positive and negative results can occur, and results must be considered in relation to risk and clinical setting. Currently, TST should be used to test for MTB infection when active disease is present.
Table 90-4 Threshold for “Positive” Tuberculin Skin Testing | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
Diagnosis of Active Tuberculosis
Although the tuberculin skin test can indicate the likelihood that an individual has been infected by MTB, history, physical examination, and other tests are required to determine whether TB is active, the anatomic sites of involvement, and the extent of disease. The diagnosis of active pulmonary TB therefore typically requires consideration of the patient’s epidemiologic risk for infection (i.e., probability of infection), clinical and radiographic presentation, the results of tuberculin skin testing, and the results of microbiologic evaluation.
Patients with a clinical presentation consistent with active pulmonary TB should be placed in respiratory isolation (or told to remain at home). Although a tuberculin skin test can be placed initially, a negative test should not be used to exclude active disease because, as noted above, between 10% to 25% of patients with active disease do not react to the skin test.48 Testing for HIV infection should be considered for all TB patients, whereas testing for the hepatitis viruses (B, C) should be done only for patients who have risk factors for those infections.
Sputum Smears and Cultures
Sputum smears and cultures are an integral part of the diagnostic evaluation in a patient suspected of having active pulmonary disease. The sputum smear is often positive in patients with cavitary disease and less often positive when noncavitary or atypical radiographic presentations occur, such as lower lobe predominance or pleural effusions, or both. Most modern mycobacterial laboratories use fluorescence microscopy with auramine-rhodamine staining to screen specimens and Kinyoun or Ziehl-Nielsen acid fast staining to confirm positive fluorescence studies. Acid fast staining is nonspecific as nontuberulous mycobacteria and other organisms (Nocardia, some Legionella) may also be acid fast–positive.
At least three specimens should be collected in sterile containers on separate days.85 These specimens may need to be induced with hypertonic saline in patients having difficulty expectorating sputum.8 The sensitivity of sputum smear for MTB ranges from 45% to as high as 92% when at least 5 mL is reliably collected.116 Because a positive smear requires a relatively high concentration of organisms, patients who are
sputum-positive are typically most infectious. AFB smears or cultures of other potentially infected sites should also be collected, if possible, to confirm concordant extrapulmonary disease. Bacteriologic yield from extrapulmonary sites is often less than from pulmonary specimens, although inclusion of histologic examination for granulomata raises the yield somewhat.
sputum-positive are typically most infectious. AFB smears or cultures of other potentially infected sites should also be collected, if possible, to confirm concordant extrapulmonary disease. Bacteriologic yield from extrapulmonary sites is often less than from pulmonary specimens, although inclusion of histologic examination for granulomata raises the yield somewhat.
Mycobacterial sputum cultures are more sensitive requiring at least a log order lower concentration of organisms to yield a positive result than the sputum smear. Hence, patients with negative sputum smears but positive cultures are typically considered less infectious. The culture can be performed on agar or egg-based media such as Lowenstein-Jensen or Middlebrook 7H10/7H11 and incubated at 37°C. The mycobacterial colonies can be identified as MTB by their appearance on media (nonpigmented corded colonies), and a variety of biochemical tests. Because MTB is a slow-growing organism, up to 8 or more weeks may be required before colonies are seen on solid media. Growth on liquid media allows mycobacteria to be detected earlier (2 to 3 weeks). BACTEC (BD Diagnostics, Sparks, MD) and Mycobacteria Growth Indicator Tube (MGIT) (BD Diagnostics, Sparks, MD) are common liquid media isolation systems, which use radiometric or calorimetric means of detection. Overall, sputum cultures are about 80% to 85% sensitive in pulmonary TB.29 Bronchoscopy with bronchoalveolar lavage may be considered in patients who are unable to raise sputum even after efforts at sputum induction with saline inhalation. Initially, positive sputum cultures should be tested for drug susceptibility for the first-line agents isoniazid, rifampin, and ethambutol. Testing for other antituberculous agents should be done if the patient has a history of prior treatment or suspected resistance to rifampin or other first-line agents.
Recently more rapid techniques have been developed for identifying MTB and certain other species of mycobacteria. Nucleic acid assay (NAA) tests use RNA probes specific for MTB or other mycobacteria (Gen-Probe MTD, Gen-Probe Inc., San Diego, CA). They can be combined with polymerase chain reaction (PCR) to amplify MTB DNA (Amplicor MTB test, Roche Diagnostic Systems, Inc., Branchburg, NJ). These assays can be completed within a day or two and have sensitivities comparable to culture systems and specificities close to 100%.75 The NAA tests are FDA-approved for sputum smear–positive patients and should, ideally, be performed on all such patients in addition to culture and drug susceptibility testing. Assays have recently been adapted to identify genetic markers of resistance—“molecular beacons”—to commonly used antituberculous drugs, thus creating the possibility of rapid drug susceptibility testing.32,111In addition, line probe assays that use a combination of PCR and reverse hybridization have been developed for both rapid diagnosis and drug resistance determination with reported high sensitivity and specificity but are not yet FDA-approved. Genetic arrays may further enhance detection and identification of resistance.11
With regard to pleural TB, characteristics of the pleural fluid may support the diagnosis. In the setting of pleural TB, pleural fluid is often devoid of mesothelial cells (<1%), and the presence of many mesothelial cells should suggest an alternative diagnosis. Additionally, the pleural fluid, if it is a chronic effusion, is often rich in cholesterol due to breakdown of cell membranes, giving it a milky appearance (pseudochylous effusion). Other diagnostic tests available for pleural TB include measurement of pleural adenosine deaminase (ADA) produced by activated T lymphocytes, IFN-γ production, pleural acid-fast bacilli (AFB) smear, culture, pleural biopsy, and polymerase chain reaction (PCR) assay to identify the presence of MTB (Tables 90-5 and 90-6).
Table 90-5 Reported Sensitivities of Diagnostic Studies in Pleural Tuberculosis | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


