Pulmonary Toxicity Related to Chemotherapeutic Agents
INTRODUCTION
Toxicities related to medications comprise a major category of iatrogenic illness. Many agents used for cancer treatment have the potential to cause pulmonary toxicity. As the horizon for treatment options has broadened with our ever-expanding understanding of biological mechanisms fundamental to neoplasia, so has the spectrum of pulmonary complications related to new therapies. Further, with availability of new therapeutic modalities, patients with cancer are living longer, and may, in their long-term survivorship, display delayed toxicities related to treatment. Consequently, for the pulmonologist, drug-induced lung disease is an area of growing complexity.
Chemotherapeutic agents, therapeutic radiation, and biological response modifiers are used in a wide range of regimens, and their use is further complicated by the use of hematopoietic support and bone marrow or stem cell transplantation. Many are, directly or indirectly, associated with pulmonary toxicity. An estimated 5% to 10% of patients undergoing chemotherapy ultimately develop therapy-related pulmonary complications.1–3 This chapter reviews the evaluation of patients with suspected chemotherapy-induced pulmonary toxicity, as well as the potential toxicities associated with specific classes of drugs.
APPROACH TO THE PATIENT WITH SUSPECTED CHEMOTHERAPY-INDUCED PULMONARY TOXICITY
The differential diagnosis of patients with cancer receiving treatment who develop pulmonary complications is often very challenging, particularly as the diagnosis of drug-induced pulmonary toxicity is typically one of exclusion. Patients most often present with nonspecific constitutional or respiratory complaints. In many cases, symptoms and physical signs may be minimal or even absent. In these situations, the only evidence of an ongoing pulmonary process may be an abnormal chest radiograph (Table 65-1).
The diagnosis of lung disease caused by chemotherapeutic agents poses a particular challenge to the clinician, as there are several complicating features inherent to the oncology patient population.
First, treatment may be given in multidrug regimens or in combination with other modalities such as radiation therapy, bone marrow transplantation, or stem cell transplantation. Assigning pulmonary toxicity to a single drug or modality within such a regimen is often impossible. Moreover, the combined toxicity of two or more drugs or a single drug with radiation therapy may exceed the individual toxicities of those drugs.
Second, patients undergoing chemotherapy are often immune suppressed, either from the malignancy itself or from myelosuppressive or immunosuppressive effects of their treatment. These patients are therefore susceptible to opportunistic infection, which may be indistinguishable radiographically from drug toxicity. This is particularly challenging, as the lung is the most common site of serious infection in patients with cancer. It has been estimated that a relative minority (5%–30%) of pulmonary complications in immunocompromised patients are actually due to drug toxicity; hence it is important to remember that infection is still the most likely culprit when pulmonary decompensation occurs. Since changing a treatment regimen may affect the chance for cure or prolongation of survival, reasonable certainty of drug-related complications necessarily involves exclusion of infection.
Third, cancers themselves may mimic lung disease. This is particularly true in cases of lymphangitic tumor spread or metastases to the lung parenchyma or pleura.
Fourth, toxicity from some drugs appears to be related to cumulative dosage levels. However, adverse reactions may occur even with a low cumulative dose, when clinical suspicion for toxicity is low.
Finally, pulmonary toxicity due to a single chemotherapeutic agent may present with several different syndromes that vary clinically, radiographically, and temporally. While a severe pulmonary reaction acutely following drug administration usually raises suspicion of drug toxicity, as patients survive for longer periods of time it is becoming increasingly clear that toxicity due to some chemotherapeutic agents may be delayed by months to even years after treatment. In such situations, clinical suspicion of drug toxicity may be low.
Monitoring for potential pulmonary toxicity in the patient undergoing chemotherapy requires ongoing clinical vigilance. Symptoms such as cough, dyspnea, or chest discomfort may be mild or even absent. Radiographic findings may be equally subtle. Even if clinical symptoms and radiographic abnormalities are present and severe, they are usually nonspecific. The possibility of adverse drug effects must be considered within the complex medical context inherent to the patient with cancer undergoing physically challenging or immunosuppressive treatment.
 PULMONARY PHYSIOLOGICAL TESTING
PULMONARY PHYSIOLOGICAL TESTING
Pulmonary physiological testing has been utilized in surveillance of patients receiving drugs with potential for pulmonary toxicity. A multitude of investigations studying the utility of pulmonary function testing (PFT) in monitoring pulmonary effects related to administration of chemotherapy have been reported, but the application of these findings to clinical management has been a subject of debate.
Various physiological abnormalities have been described, the most common of which are decreases in lung volumes and diffusing capacity for carbon monoxide (DLCO). Patients receiving chemotherapy who are monitored serially by PFT frequently demonstrate physiological abnormalities in the absence of clinical signs of toxicity.4–6 Abnormalities in DLCO in particular have been thought by some to be indicative of early-onset, drug-related pulmonary injury. Most such studies have been performed in patients receiving bleomycin, busulfan, or carmustine. Discontinuation of drug, with or without initiation of treatment, including corticosteroids, typically results in improvement. Whether early intervention based on DLCO abnormalities in the absence of clinical symptoms decreases the likelihood of long-term pulmonary impairment related to toxicity is unclear. Conversely, in situations of clinically evident drug toxicity accompanied by PFT abnormalities, withdrawal of culprit therapy may not be paralleled by improvement in physiological measurements. For example, in a study examining pulmonary function in 116 long-term (5–13 years after treatment) survivors of Hodgkin disease in Norway, nearly 30% of patients had exertional dyspnea with associated pulmonary function abnormalities.7 Multivariate analysis of these patients identified chemotherapy with a combination of bleomycin and anthracyclines as the sole significant predictor of lung function impairment. In all patients in whom drug toxicity is of concern, consideration must be given to the possibility that discontinuation of a specific treatment might result in substitution of less effective therapy.
A number of factors further complicate the practice and interpretation of PFT in the oncology population. Many physiological parameters are effort dependent. The ability of a patient to consistently perform test maneuvers may be affected by weakness, pain, or the use of analgesic or sedating medications. Reproducibility of results therefore may be a significant concern in patients whose functional status and strength are impaired by their malignancy or its treatment. Many patients have anemia induced by malignancy, medication, or chronic illness. Since DLCO is affected by hemoglobin concentration, it is critical that appropriate corrections for anemia be made. Patients with cancers may also be subject to processes other than drug toxicity that will affect PFT results. Primary pulmonary malignancy, metastatic lung disease, infection, thoracic or abdominal surgical procedures, and a host of other clinical situations may all independently cause variation in physiological measurements. Therefore, identifying pulmonary physiological abnormalities specific to drug effect may prove very difficult.
Ultimately, even though the predictive value of baseline or serial PFT remains unclear, most clinicians will continue to rely on such testing as screening and monitoring tools. Though there are no definitive data that toxicity can be averted by physiological monitoring, we are limited by the absence of other means of identifying toxicity early enough to prevent severe pulmonary disease. Though the presence of subclinical abnormalities does not imply that patients will develop irreversible lung disease, these abnormalities may dictate closer monitoring, or even the withdrawal of drug. Conversely, normal physiology cannot predict abrupt toxicity that may produce profound pulmonary injury. As always, medical decisions based on pulmonary physiological findings must be made in the context of the patient’s clinical situation as a whole.
 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
Given the potential impact of pulmonary drug toxicity on a patient’s present and future cancer treatment, it is important to establish this diagnosis as firmly as possible. Thoughtful and judicious use of invasive procedures plays an important role in that evaluation.
The approach to the cancer patient in whom drug toxicity is suspected should parallel the approach to any immunocompromised patient with diffuse or localized lung disease (see Chapter 123). Because clinical features are usually not specific, sampling of respiratory tract secretions and/or lung tissue may be critical to this evaluation. Direct sputum examination or culture may suggest specific pathogens or may be diagnostic of infections such as invasive fungal disease, Pneumocystis jiroveci pneumonia, or tuberculosis. In the absence of diagnostic sputum findings, invasive procedures may be necessary. Fine-needle aspiration of the lung may be useful with focal lesions. However, the utility of this procedure in diffuse lung disease is relatively low. This is particularly problematic for patients with drug-induced pulmonary toxicity, which often presents with a diffuse interstitial pattern on chest radiograph. Fiberoptic bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy has become central to the evaluation of both diffuse and localized lung disease in the immunocompromised host. The procedure is associated with a low rate of major complications. Diagnostic yield varies widely, reflecting the broad range of disease processes that can involve the lung in the immunocompromised patient. Highest diagnostic yields are obtained in patients with infections; lower yields are seen in interstitial inflammatory processes, which may include toxicity from drugs. However, even in situations in which a specific etiology is not identified, exclusion of infection by bronchoscopy often provides clinically useful information.
Open or thoracoscopic lung biopsy is associated with the highest diagnostic yield and can be performed with low complication rates even in critically ill patients. If drug-induced pulmonary injury is suspected, surgical biopsy may be necessary to definitively exclude other causes of lung disease.
The evaluation of a patient in whom chemotherapy-related pulmonary toxicity is a consideration clearly presents significant challenges. Clinicians must be vigilant in the evaluation and management of patients receiving chemotherapeutic regimens. An awareness of potential iatrogenic complications related to drug therapy is, therefore, essential.
CYTOTOXIC ANTIBIOTICS
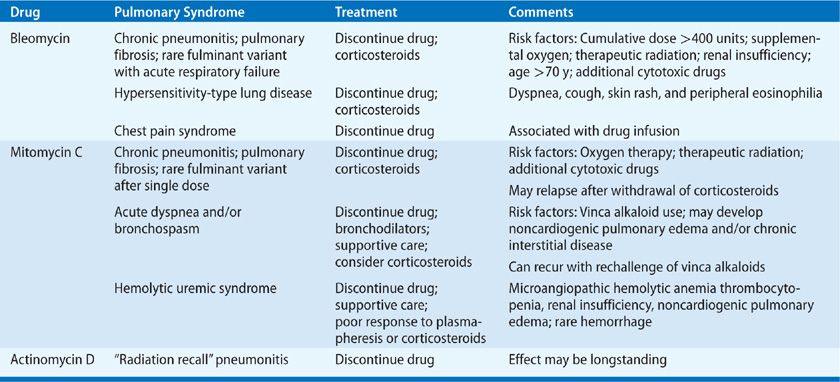
A variety of cytotoxic antibiotics have been associated with pulmonary toxicity (Table 65-2). Important examples are discussed below.
 BLEOMYCIN
BLEOMYCIN
Bleomycin, a cytotoxic antibiotic produced by Streptomyces vermiculus, is used in the treatment of various malignancies, including lymphomas, germ cell tumors, and squamous cell cancers of the head and neck.8 Unfortunately, it has significant potential for pulmonary toxicity due to relative lack of the inactivating enzyme bleomycin hydrolase in the lungs.9 The most severe complication is interstitial pneumonitis progressing to fibrosis and respiratory failure, though there are also less severe syndromes, including organizing pneumonia and hypersensitivity pneumonitis.10
The pulmonary toxicities of bleomycin have been studied extensively, primarily in animal models. Endothelial injury via oxidative stress is the sentinel event, followed by the influx of inflammatory cells (predominantly macrophages, neutrophils, and lymphocytes), development of perivascular edema, elaboration of inflammatory cytokines, and, ultimately, fibroblast activation and fibrosis (Fig. 65-1).11 A variety of mediators have been implicated in bleomycin-induced lung injury, including tumor necrosis factor α (TNFα), transforming growth factor-β (TGF-β), interleukin-6 (IL-6), and interleukin-1 (IL-1).10 Human studies have demonstrated activation of in vitro alveolar macrophages in response to bleomycin,12 and patients treated with bleomycin for testicular cancer demonstrate a rise in serum TNFα 3 to 24 hours after drug administration.13 The continued expression of TNFα and IL-1 may predispose to the production of TGF-β and promotion of dysregulated collagen production and fibrosis. Animal studies have shown that bleomycin-induced pulmonary toxicity can be ameliorated or prevented by antibodies to TNFα and TGF-β, or receptor antagonists to IL-1; knockout of the TNFα receptor has also demonstrated protective effects.14–16 Bleomycin also induces direct free-radical damage after oxidation of the bleomycin-Fe (II) complex; this effect has been mitigated by iron depletion with chelators both in vitro and in vivo.10
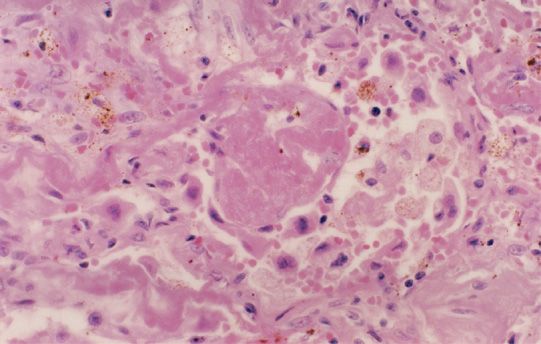
Figure 65-1 Lung biopsy specimen from a patient with clinical and radiographic evidence of bleomycin-induced pulmonary toxicity shows drug effect with acute and chronic changes. The alveolus contains an exudate of fibrin, which is undergoing organization and is surrounded by alveolar macrophages. The large and atypical cells are markedly reactive alveolar type II pneumocytes. The alveolar wall itself is scarred with collagen deposition by the spindle-shaped fibroblasts. (Used with permission of Dr. Darryl Carter, Professor of Pathology, Yale University School of Medicine.)
The incidence of bleomycin-induced pneumonitis varies from 6% to 18%, though this figure may be higher depending on the criteria used for diagnosis and the presence of other chemotherapeutic agents in a multidrug regimen; overall mortality is estimated at 3% or less, depending on risk factor subgroup.10,17
Several risk factors for severe bleomycin-induced pneumonitis have been identified: (1) Toxicity appears to correlate with higher cumulative dosages. While fatal injury has been observed after administration of <100 units, there is a significant escalation in toxicity with total doses over 400 units, and severe toxicity develops in 20% of patients receiving >500 units.18 (2) Exposure to high concentrations of supplemental oxygen, particularly in the setting of general anesthesia, may create a synergistic toxic effect. This is primarily based on animal data, including a study which demonstrated a 75% increase in mortality in hamsters treated with bleomycin and 70% oxygen for 72 hours versus bleomycin alone.19 As a result, high fractions of supplemental oxygen are generally avoided whenever possible in clinical practice, and the evidence substantiating human risk has been largely anecdotal. There have also been case reports of recrudescence in patients exposed to even modest levels of supplemental oxygen several months after bleomycin exposure, so caution in limiting oxygen exposure for at least 6 months after treatment appears warranted.20 (3) Thoracic irradiation prior to, concomitant with, or subsequent to, bleomycin administration may be associated with an increase in toxicity. This “radiation recall” may extend outside the original port of irradiation, and may last for years after bleomycin therapy. More recent data suggests that the augmented risk from consolidative radiation therapy may be attenuated if there is an interval of at least 28 days between chemotherapy and radiation.21 While there is still certainly an increase in pulmonary symptoms during treatment for patients receiving combined therapy, and patients must be closely monitored, long-term sequelae may be less pronounced than originally feared.22 (4) Since bleomycin is excreted by the kidneys, and is often coadministered with potentially nephrotoxic agents, decreased creatinine clearance below 35 mL/min is associated with increased risk of toxicity.23 (5) The risk for pulmonary toxicity rises proportionately for every decade after 30 years, and patients over 70 years of age are at particular risk.24 (6) Concurrent use of other chemotherapeutic regimens, including gemcitabine, cisplatin, and drugs in the ABVD regimen, may result in synergistic toxicity.21,22 Though these effects have not been clearly reproducible in all cases, convention has been to reduce bleomycin dosage in drug regimens in which this synergy is a concern. (7) Smoking appeared to confer an increased risk of bleomycin toxicity in one study,25 however this has not been confirmed in other studies, and may be confounded by the presence of other risk factors. (8) Similarly, though there have been case reports of increased incidence of bleomycin toxicity in patients receiving granulocyte colony–stimulating factor (G-CSF), likely due to increased cytokine induction, larger series have not clearly demonstrated a relationship.26
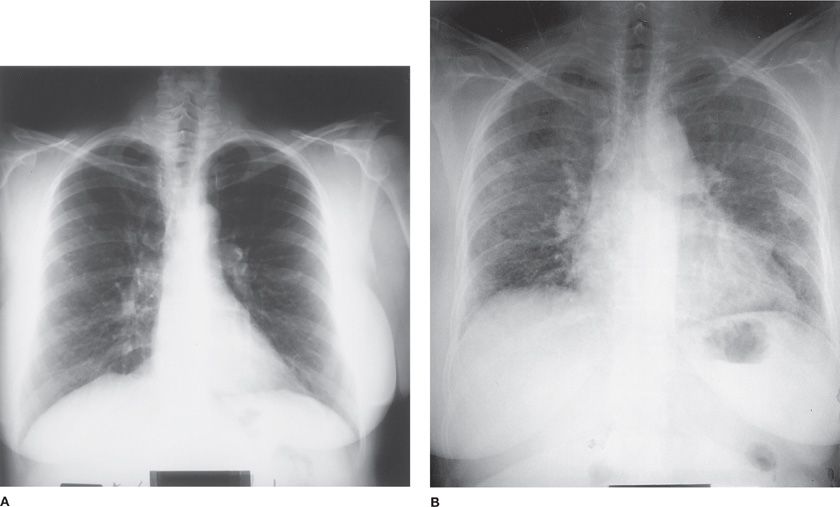
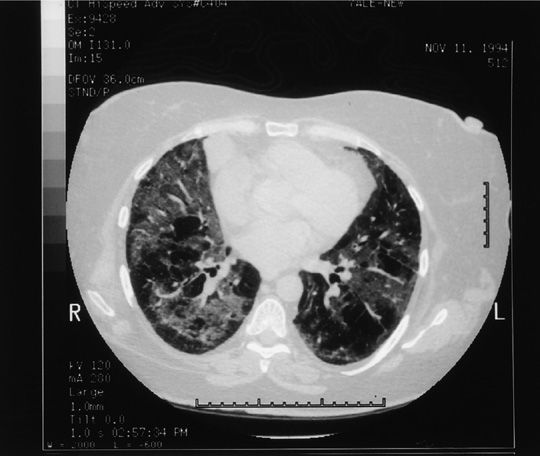
The clinical presentation of bleomycin-induced pneumonitis is usually subacute and insidious, occurring within a few weeks to 6 months after treatment.10 A more fulminant presentation with acute respiratory failure has been reported but is far less common. Patients generally present with dyspnea, nonproductive cough, and low-grade fever, though some patients may be asymptomatic. Substernal or pleuritic chest pain occurs, but is infrequent. Common physical findings include hypoxemia and fine bibasilar crackles; rhonchi and a pleural rub may also develop.27 Chest radiograph usually shows bilateral reticular or fine nodular infiltrates with a basilar predominance, often beginning at the costophrenic angles (Fig. 65-2A,B). Loss of lung volume with diaphragmatic elevation is also commonly seen. However, various radiographic patterns including alveolar infiltrates, lobar consolidation, organizing pneumonia, asymmetric lung involvement, pneumothorax, pneumomediastinum, and even lung nodules have been described. Computed tomographic (CT) scanning, particularly high-resolution computed tomography (HRCT) scanning, is more sensitive in the evaluation of radiographic abnormalities and may be useful in patients who have spirometric or clinical evidence of toxicity but negative chest radiographs (Fig. 65-3); CT scanning provides more accurate assessment of anatomic distribution of disease that correlates well with lung function impairment.28 Most patients receiving bleomycin demonstrate a decrease in DLCO over the course of therapy, though only a small percentage of these patients will go on to manifest clinical signs of pulmonary toxicity, which is typically also associated with restrictive ventilatory defect.29
Figure 65-2 Posteroanterior chest radiographs of a 56-year-old woman with cervical carcinoma (A) before and (B) after chemotherapy with a bleomycin-containing regimen. Note the decrease in lung volume and diffusely increased interstitial lung markings in the postchemotherapy radiograph.
Figure 65-3 Chest computed tomography (CT) scan of same patient as in Figure 65-2, taken at the time of radiograph in Figure 65-2. Note the patchy distribution of bilateral infiltrates, whose extent is clearly delineated by CT.
Bleomycin may also cause an acute hypersensitivity syndrome of dyspnea, cough, and rash immediately following administration of drug. Lung biopsy in these cases shows eosinophilic infiltration, and changes consistent with hypersensitivity pneumonitis; peripheral eosinophilia may also be observed. These cases show a particularly favorable response to steroid therapy.30 An acute chest pain syndrome has also been reported in 1% of patients; it occurs during infusion, resolves with termination of drug, and does not appear to predict other pulmonary toxicity from bleomycin.31
Discontinuation of drug is recommended for patients with clinically significant bleomycin-induced toxicity, and may be sufficient treatment for individuals with mild presentations. For more significant disease, corticosteroids are usually given in the range of 60 to 100 mg of prednisone per day for 4 to 8 weeks, with slow taper over the following 4 to 6 months guided by clinical stability of the patient. It should be noted that the steroid responsiveness of bleomycin-induced pulmonary toxicity is highly variable, with more responsive cases likely representing hypersensitivity pneumonitis or organizing pneumonia.10 Improvement often occurs within weeks of drug cessation, but complete resolution may take up to 2 years; patients may be left with residual radiographic and/or physiological abnormalities, particularly if fibrosis has developed. Though a number of promising agents have been explored in animal models, none have yet been shown to be effective in humans.
 MITOMYCIN C
MITOMYCIN C
Mitomycin C is an alkylating agent derived from Streptomyces caespitosus. It is used in multidrug regimens for multiple solid organ malignancies including non–small-cell lung, breast, gastric, pancreatic, cervical, prostate, and bladder cancers.32 The incidence of pulmonary toxicity due to mitomycin is variably reported between 2% and 38% (with clinically relevant toxicity likely <10%), and appears to be potentiated by concurrent administration of other agents, particularly vinca alkaloids.32 Though typically associated with intravenous therapy, cases of significant pulmonary toxicity have been reported in patients receiving both intravesicular and intraperitoneal mitomycin C.33,34 Mitomycin C lung injury presents with multiple distinct pulmonary syndromes, including interstitial pneumonitis and fibrosis, bronchospasm, acute lung injury, thrombotic microangiopathy, venoocclusive disease with pulmonary hypertension, and pleural disease.
The most common form of mitomycin-induced lung toxicity is a chronic pneumonitis with pulmonary fibrosis similar to that seen with bleomycin; it similarly appears potentiated by exposure to supplemental oxygen and radiation.35 Some studies have suggested a dose–response effect, with increased risk of toxicity after cumulative doses ranging from 20 to 39 mg/m2, though this finding has not been consistently reproducible.28,29 The exact mechanism of injury is unknown, though several have been proposed, including lipid peroxidant injury, hypersensitivity reactions, or immune complex–mediated disease.36 Pulmonary toxicity usually occurs after 2 to 12 months of therapy, though a more fulminant form of acute lung injury has been reported after a single dose.36
Clinically, patients present with a subacute syndrome of cough and progressive dyspnea, often with fatigue and sometimes with pleuritic chest pain.37 Fever is less common. Chest radiographs usually show bilateral interstitial infiltrates, occasionally with alveolar or fine nodular patterns. PFTs demonstrate a restrictive pattern with impairment in DLCO, though degree of impairment correlates poorly with prognosis.6 Histologically, biopsy specimens show mononuclear cell infiltration, alveolar-lining cell hypertrophy, collagen deposition, and alveolar septal thickening; type II pneumocyte enlargement and lymphocytic or eosinophilic infiltration have also been described. This syndrome may respond to discontinuation of drug and institution of corticosteroids at an initial dose of 60 mg/d tapered over a 4- to 6-week period; patients may demonstrate relapse once steroids are discontinued.37
The second syndrome of mitomycin-induced pulmonary toxicity is primarily seen in patients who have also received vinca alkaloids. While drugs of this latter category (vinblastine, vinorelbine, and vindesine) confer little in the way of risk of pulmonary toxicity when used alone, they may precipitate a syndrome of acute pulmonary toxicity when given concurrently with or subsequent to administration of mitomycin C.38 Patients present with rapid onset of dyspnea or bronchospasm hours to weeks after exposure; symptoms generally abate in 12 to 24 hours with cessation of drug, supportive care, and bronchodilators. In some cases, however, bilateral interstitial infiltrates or noncardiogenic pulmonary edema may also develop, and patients may go on to develop chronic interstitial lung disease with permanent physiological impairment.39 Rechallenge with vinca alkaloids alone, independent of mitomycin C, can also result in recrudescence of symptoms.38
The third syndrome of mitomycin C toxicity is a microangiopathy, with a presentation similar to thrombotic thrombocytopenic purpura and hemolytic uremic syndrome (TTP–HUS); in the case of mitomycin C microangiopathy, it is also associated with acute lung injury and respiratory failure in 50% of cases.40 Pulmonary alveolar hemorrhage in this setting has also been described.41 The mechanism of toxicity appears related to endothelial injury in the pulmonary vasculature. Unfortunately, prognosis is poor, and patients with chemotherapy-induced TTP–HUS respond poorly to the plasma exchange and corticosteroids that are the mainstay of therapy; there are case reports of successful treatment with rituximab, though it should be noted that these patients did not have respiratory failure.42,43
In addition, mitomycin C has been implicated in two cases of fatal pulmonary hypertension caused by pulmonary venoocclusive disease in patients with non–small-cell lung cancer (NSCLC) treated prior to surgical resection.44 There have also been reports of pleural toxicity, with exudative effusions and pleural fibrosis.6
 ACTINOMYCIN D
ACTINOMYCIN D
Actinomycin D is an older antitumor antibiotic derived from Streptomyces, which is still used in the treatment of Ewing sarcoma, rhabdomyosarcoma, Wilms tumor, and gestational choriocarcinomas. While this drug is not often associated with primary lung toxicity, it may potentiate “radiation recall” pneumonitis in patients who have received prior thoracic irradiation.45
ALKYLATING AGENTS
Alkylating agents (Table 65-3) have been implicated in chemotherapeutic agent–related lung disease. Important examples are described below.
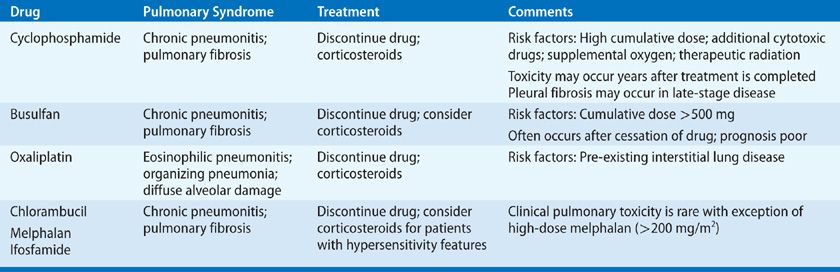
The chemotherapeutic properties of the alkylating agents result from the formation of covalent linkages (alkylation) of DNA components.46 Nitrogen mustards are the prototypic alkylating agents and were the first drugs to be used as modern cancer chemotherapy, but many other drugs also exert antineoplastic effects by alkylation. Alkylating agents that have been associated with pulmonary toxicity include derivatives of nitrogen mustards (cyclophosphamide, melphalan, chlorambucil, ifosfamide), alkyl sulfonates (busulfan), platinum-based therapies (oxaliplatin), and the nitrosoureas (carmustine/BCNU, lomustine/CCNU). The nitrosoureas are considered in a separate section below.
 CYCLOPHOSPHAMIDE
CYCLOPHOSPHAMIDE
Cyclophosphamide is widely used in the treatment of many malignancies, including lymphomas, breast and ovarian cancers, and a variety of other solid tumors. It may be used as part of myeloablative conditioning regimens prior to bone marrow or peripheral blood stem cell transplantation. It is also used alone or in combination with corticosteroids in the treatment of autoimmune diseases and systemic vasculitides.3 The overall incidence of cyclophosphamide-induced lung injury is less than 1%, though as with other agents, increased pulmonary toxicity may occur in the setting of radiation therapy, oxygen supplementation, or combination treatment with other cytotoxic agents.47
Cyclophosphamide is administered as an inactive prodrug that is metabolized by the liver, and to lesser extent, in the lung, to 4-hydroxycyclophosphamide, phosphoramide mustard (responsible for alkylation and DNA cross-linking), and acrolein (responsible for the hemorrhagic cystitis which can complicate therapy).48 Though the exact mechanism of cyclophosphamide-induced injury to the lung is unknown, in vitro models suggest contributions from oxidative stress, and upregulation of TGF-β, increase in collagen synthesis and, ultimately, fibrosis.49 Cyclophosphamide toxicity lacks a clear dose–response relationship in humans, possibly due to genetic variations in drug metabolism.50 In addition, the pharmacokinetics of both the inactive parent compound and the active alkylating derivative can be affected by variations in the cytochrome P450 superfamily of enzymes, as well as by interactions with other drugs. Therefore, functionally higher exposure to active drug may occur in the setting of substances that induce hepatic enzyme activity, such as rifampin, phenytoin, and alcohol, or with decreased renal clearance.
As with a number of other chemotherapeutic agents, cyclophosphamide-induced pulmonary toxicity may present either early in the course of treatment or in a delayed, progressive fashion that may begin years after treatment is completed. In cases where pulmonary symptoms occur long after exposure, an association with the drug may be difficult to identify. Clinical features are nonspecific, including nonproductive cough, dyspnea, fatigue, and fever. Occasionally, patients are asymptomatic but are discovered to have radiographic abnormalities compatible with drug toxicity. Chest radiographs and CT scans usually show evidence of bilateral interstitial lung disease (either bilateral reticular or nodular markings with ground-glass opacities or, later, fibrosis) but may also show pleural fibrosis in late-stage disease.51 This latter radiographic finding may be helpful in distinguishing cyclophosphamide-associated interstitial lung disease from the idiopathic interstitial pneumonias.
Histological findings in the lung are not specific. Lung biopsy in these patients is primarily useful for exclusion of other identifiable causes of interstitial lung disease in immunocompromised patients, including infection and malignancy. When used to treat nonneoplastic lung disease, the distinction between underlying lung disease from a systemic syndrome and lung disease exacerbated by drug toxicity is often very difficult to delineate. When cyclophosphamide is used as a chemotherapeutic agent, its identification as the specific etiology of lung injury may also be difficult, as it is rarely used alone, pinpointing specific toxicity to a single agent may be impossible. Like other agents, cyclophosphamide may have synergistic toxicity with therapeutic thoracic radiation, high levels of supplemental oxygen, and other chemotherapeutic drugs with potential for lung injury.52
Cyclophosphamide-induced lung injury may cause significant morbidity, and a high clinical suspicion for pulmonary toxicity should result in prompt discontinuation of the drug. Early-onset pneumonitis can occasionally be fatal, but when recognized quickly, prognosis is generally favorable and recovery, though slow, is expected for most individuals. Discontinuation of the drug alone may be sufficient, though most patients also receive glucocorticoid therapy. The optimal regimen and magnitude of benefit in these cases remain unclear.53 Unfortunately, late-onset toxicity follows an irreversible and progressive course, which appears steroid unresponsive; mortality due to progressive respiratory failure exceeds 60%.51
 BUSULFAN
BUSULFAN
Busulfan, previously used as a treatment for chronic myelogenous leukemia (CML) prior to the advent of oral tyrosine kinase inhibitors, is now mainly used as a component of conditioning regimens for bone marrow and stem cell transplantation. Toxicity may develop within weeks of exposure, but is more typically insidious, with the average onset of symptoms more than 3 years after treatment.3 Estimates of frequency vary widely, with an average of approximately 6%.54 Because of the indolent nature of CML, patients were often treated for months to years with busulfan. Though it was generally well tolerated in this setting, patients receiving a cumulative dose above 500 mg appeared to be at higher risk for pulmonary complications.55
Currently, busulfan is used in combination with other chemotherapeutic agents in conditioning regimens prior to bone marrow and stem cell transplantation. Pulmonary complications in this situation are not uncommon though it is difficult to clearly attribute toxicity to busulfan rather than other causes including infection (notably cytomegalovirus), radiation therapy, and other drugs (particularly etoposide).56,57 In long-term comparisons of patients receiving busulfan and cyclophosphamide versus total body irradiation prior to allogeneic transplantation, bronchiolitis obliterans was far more frequent in the former group (26% vs. 5%), while rates of pneumonitis were similar; this raises the concern that the main source of pulmonary toxicity in busulfan-based conditioning regimens may be an increase in chronic pulmonary graft versus host disease.58
Symptoms of busulfan lung injury usually present insidiously, weeks to years after exposure, with cough, progressive dyspnea, fever, fatigue, and weight loss. Chest radiographs typically show bilateral interstitial infiltrates with a basilar predominance. Pathological findings are consistent with other cytotoxic drug–induced pulmonary injuries, with type II pneumocyte hyperplasia, dysplasia, and desquamation into alveolar spaces. Fibroblast proliferation, collagen deposition, and fibrosis are usually evident. Desquamation and accumulation of alveolar debris can be severe in some cases, yielding a pattern similar to pulmonary alveolar proteinosis; unfortunately, total lung lavage is usually ineffective in such cases.3,59
There is no specific treatment for busulfan-induced pulmonary injury, except withdrawal of the drug. However, due to the delayed nature of presentation, patients are often no longer on therapy by the time toxicity is detected; thus, treatment is largely supportive. Though some spontaneous improvement may occur, when there is clinically evident busulfan-induced pulmonary toxicity the prognosis for recovery is generally poor. Corticosteroids have anecdotally been reported to be of benefit but, as with most chemotherapeutic agents, no prospective studies are available.3 Given the possibility of late-onset pulmonary toxicity, it seems prudent that long-term follow-up of recipients of bone marrow or peripheral blood stem cell transplants with busulfan-based conditioning regimens should include pulmonary evaluation. However, guidelines for identification or treatment of pulmonary toxicity in this situation are lacking.
 OTHER ALKYLATING AGENTS
OTHER ALKYLATING AGENTS
Chlorambucil and melphalan are both slow-acting nitrogen mustards. Chlorambucil has an important role in the treatment of lymphoreticular malignancies including chronic lymphocytic leukemia (CLL) and has also been used in the treatment of nonneoplastic diseases, such as rheumatoid arthritis and sarcoidosis. Though pulmonary toxicity is less common than with other alkylating agents, occurring in less than 1% of patients, mortality from irreversible fibrosis when it occurs exceeds 50%.60 As with busulfan, chlorambucil may be administered over a prolonged time in the treatment of CLL, but there does not appear to be a clear relationship between toxicity and either cumulative dose or duration of therapy. The number of cases of reported chlorambucil pulmonary toxicity is relatively small, thus no distinct clinical pattern has emerged. In cases of chlorambucil-related interstitial pneumonitis, BAL has demonstrated a CD8+ T cell alveolitis suggestive of hypersensitivity reaction.61 Given the possibility of hypersensitivity pneumonitis, clinical suspicion should prompt immediate discontinuation of the drug, and administration of corticosteroids can be considered in patients with progressive pulmonary disease.
Melphalan has traditionally been used in the treatment of multiple myeloma, but, like other alkylating agents, it is now used to treat a variety of malignancies. High-dose melphalan (200 mg/m2 or more) is used in conditioning regimens prior to stem cell transplantation and has been associated with fatal pneumonitis and fibrosis.62 Since large series of such patients are not available, the incidence of pulmonary toxicity associated with high-dose melphalan given in these situations is not known. As these types of treatments become more widely available, new data should define whether pulmonary toxicity related to melphalan or other alkylating agents is indeed more prevalent than has been historically appreciated.
Ifosfamide is an alkylating agent that is structurally related to cyclophosphamide. It is used in the treatment of lymphoma and acute and chronic leukemias, as well as in solid tumors including sarcomas, ovarian cancer, and breast cancer. Dose limitation is usually related to bladder toxicity. Clinically evident ifosfamide-induced pulmonary toxicity appears to be rare and typically presents as interstitial pneumonitis.63 It has also been described as a cause of acquired methemoglobinemia; this should be considered in the differential for patients on ifosfamide presenting with dyspnea, cyanosis, or altered mental status.64
Oxaliplatin is a platinum-derivative cytotoxic agent primarily used as part of multidrug regimens with 5-fluorouracil and leucovorin (FOLFOX) for treatment of colorectal cancer, as well as for treatment of pancreatic, breast, ovarian, and NSCL cancers. Though pulmonary complications are relatively rare, variable patterns of lung toxicity have been reported with FOLFOX regimens, including eosinophilic pneumonia, organizing pneumonia, and diffuse alveolar damage.65–68 The timing, severity, progression, and prognosis of individual cases have been heterogeneous, probably owing to the diversity in pathological mechanisms. Some cases have demonstrated complete resolution with withdrawal of drug, with or without administration of corticosteroids, but others have nonetheless progressed rapidly to respiratory failure and death.66,68 In some cases, patients were successfully rechallenged with 5-fluorouracil and leucovorin, indicating oxaliplatin as the likely culprit in the original drug reaction.65,67 Mechanism of toxicity is still poorly understood, and likely multifactorial; glutathione depletion has been suggested based on the mechanistic role in oxaliplatin-induced hepatic injury, and an anecdotal case report of an individual who improved when treated with a combination of corticosteroids and N-acetylcysteine.69,70 Patients with pre-existing interstitial lung disease, even when subclinical, may be at increased risk for oxaliplatin toxicity, progression of underlying interstitial disease, or both.70 Individuals with baseline physiological and radiographic abnormalities, even in absence of symptoms, should be monitored carefully, and the threshold for withdrawal of drug and trial of corticosteroids should be low. It is unclear whether prophylactic N-acetylcysteine might be of benefit in these cases.
ANTIMETABOLITES
Antimetabolites are associated with lung injury (Table 65-4). Representative examples are discussed below.