Pulmonary Pharmacotherapy
A wide spectrum of therapeutic agents is currently employed in the treatment of respiratory disorders, including obstructive lung diseases. This chapter reviews the rationale for, and clinical use of, these agents in current clinical practice.
DRUG DELIVERY
Inhaled drug administration is preferred for many medical conditions. Advantages of inhaled drug administration include rapid onset of action and the ability to deliver small drug doses directly to the lungs, minimizing systemic drug exposure. Compressed air nebulizers have been in use for more than 150 years; the first metered-dose inhaler (MDI) became available in the 1950s, followed by the first dry powder inhaler (DPI) in the 1960s.1 Many new and innovative devices have been marketed as the result of the phase-out of chlorofluorocarbon-containing MDIs.2
Device selection depends on drug–device availability, patient characteristics (e.g., age, cognitive function, manual dexterity), and patient preference (Tables 145-1 and 145-2).3,4 MDIs and nebulizers share universal designs; other drug delivery devices are unique, individually patented devices.

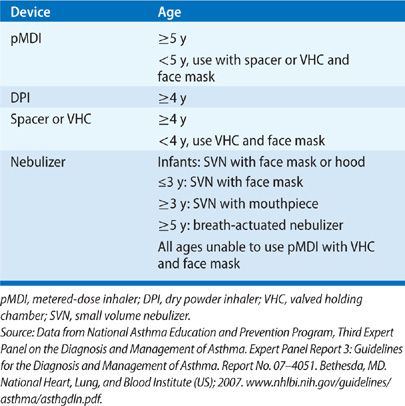
MDIs are small portable devices that protect the medication from contamination; they are difficult to use correctly. Common drug administration errors include improper timing of actuation and inspiration and failure to inspire slowly and deeply. MDI accessory devices (e.g., spacers, valved holding chambers) decrease oropharyngeal drug deposition and reduce the need for precise “press and breathe” timing, but they are bulky, add to the cost of therapy, and must be regularly cleaned to reduce bacterial contamination and electrostatic charges within the chamber. The Respimat device is a unique, multidose liquid inhaler that uses spring-loaded energy to generate a fine aerosol mist.5
DPIs are available as premetered devices that contain or accept single doses or as bulk reservoir devices. DPIs are breath actuated, requiring rapid and forcible inhalation to aerosolize and deliver the dose; most require inspiratory flow rates of 30 to 60 L/min. Young children, the elderly, and patients with reduced lung function (e.g., during an acute exacerbation) may be unable to generate adequate inspiratory flow rates.
Nebulizers aerosolize a solution or suspension by one of the three mechanisms: compressed air or oxygen (jet nebulizers), high-frequency ultrasonic energy (ultrasonic nebulizers), and vibrating mesh. Suspensions can only be aerosolized with jet nebulizers. Nebulizers are more expensive, require more dose preparation, and take more time to deliver a dose than other drug delivery devices. However, they require less patient cooperation and may be used by patients of all ages and abilities.
BRONCHODILATORS
Bronchodilation produced by β-Adrenergic agonists, muscarinic antagonists, and methylxanthines is a major component of the pharmacologic management of obstructive airway diseases. Other drugs (e.g., magnesium) have been used clinically for bronchodilation.
 β-ADRENERGIC AGONISTS
β-ADRENERGIC AGONISTS
The β-Adrenergic agonists mimic the actions of norepinephrine at neuroeffector and synaptic junctions. Norepinephrine is the major neurotransmitter in the sympathetic nervous system; therefore, this class of drugs is referred to as adrenergic agonists or sympathomimetics. The two major types of adrenergic receptors are the α and β receptors; at least two α and three β receptor subtypes have been identified.
β-Adrenoceptor stimulation relaxes smooth muscles via cyclic-3′5′-adenosine monophosphate (cAMP)-dependent and cAMP-independent mechanisms. Adrenergic receptor stimulation catalyzes the conversion of adenosine triphosphate (ATP) to cAMP by activating adenyl cyclase, a cofactor in the production of cAMP. The increase in cAMP triggers the intracellular events that mediate pulmonary and extrapulmonary responses. β-Adrenoceptor stimulation also activates large-conductance, calcium-activated potassium channels in plasma membranes independent of cAMP.6
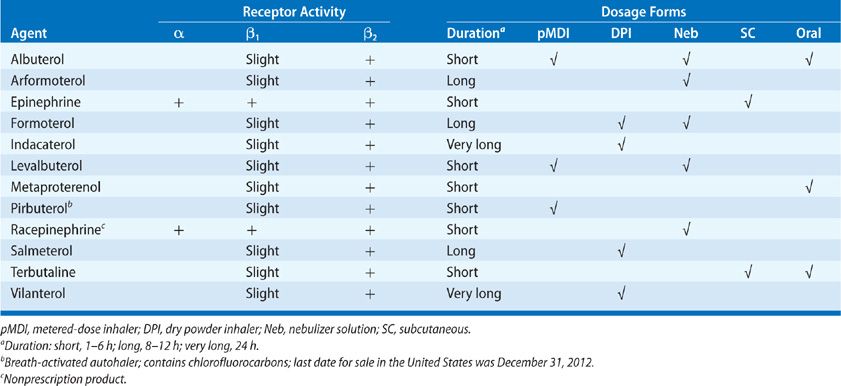
The β-Adrenergic agonists (Table 145-3) are indicated in the treatment of bronchospasm associated with acute and chronic asthma, bronchitis, emphysema, exercise, and other obstructive pulmonary diseases. Selection of a specific agent and route of administration depends on underlying patient risk factors and the receptor specificity of the drug.
Pharmacology
Adrenergic receptor stimulation produces a wide range of responses, depending on the effector organ and the specific receptor.7 Although bronchial smooth-muscle relaxation results from β2-Adrenergic receptor stimulation, none of the currently marketed agonists are completely specific for β2-Adrenergic receptors. The α-Adrenergic receptor is generally associated with constrictor/contractor responses, including constriction of arteries and veins and contraction of the uterus, radial and sphincter muscles of the iris, urinary bladder, and stomach sphincters. β1-Adrenergic receptor stimulation increases heart rate, atrial and ventricular contractility, and cardiac conduction velocity. Effects from β2-Adrenergic receptor stimulation include relaxation of bronchial and uterine smooth muscle, dilatation of arteries and veins, and several metabolic effects, including glycogenolysis, gluconeogenesis, and induction of hepatic pancreatic β-cell secretion.
Structure–Activity Relationships
The parent compound for the adrenergic agonists, phenylethylamine, consists of a benzene ring and an ethylamine side chain. Substituents can be added to the α or β carbons of the ethylamine side chain, the terminal amine group, or one or more of the carbons in the aromatic ring.8 The addition of hydroxyls at positions 3 and 5 of the aromatic ring (e.g., metaproterenol and terbutaline) promotes oral bioavailability. Large substituents added to the amino group promotes β2-Adrenergic receptor selectivity and favors a long duration of action (e.g., salmeterol, formoterol, indacaterol, vilanterol).
At least two theories have been proposed to explain the long duration of action of the long-acting and ultra long–acting β2-Adrenergic agonists (LABAs).9,10 The exosite theory states that a portion of the large side chain on the terminal amine binds to a site separate from, but close to, the β2-Adrenergic receptor, keeping the active portion of the structure available for binding and rebinding to the β2-Adrenergic receptor. The diffusion mucokinetic model states that the plasma membrane serves as a drug reservoir. However, neither theory fully explains the long duration of action of salmeterol, formoterol, indacaterol, or vilanterol, suggesting additional mechanisms.
Enantiomers
Albuterol and formoterol are 1:1 mixtures of (R)- and (S)-enantiomers. The therapeutic effect of albuterol comes from the (R)-enantiomer; the (R)-enantiomer is marketed separately as levalbuterol. Early in vitro studies suggested that (S)-albuterol might antagonize the effects of (R)-albuterol, but levalbuterol has not been demonstrated to be clinically superior to racemic albuterol.11 The (R)-formoterol enantiomer, marketed as arformoterol, is 1000-fold more potent in β2-recetor binding affinity than the (S)-enantiomer.12 The racemic mixture is marketed as formoterol. Formoterol and arformoterol are equally effective bronchodilators with similar side effect profiles.13
Drug Delivery
The β-Adrenergic agonists may be administered systemically or by inhalation; however, not all drugs are marketed in every dosage form (Table 145-1). Systemic dosage forms include oral, subcutaneous, and intravenous preparations. Systemic administration decreases the β2-Adrenergic receptor selectivity of the drug due to exposure to various metabolic enzymes, including catechol-o-methyl transferase, monoamine oxidase, and sulfatase. These enzymes change the chemical structure of the drug, decreasing the β2-Adrenergic receptor selectivity.
Inhaled β-Adrenergic agonists are available in several dosage forms, including wet aerosols, aerosols from MDIs, and dry powder forms. Historically, nebulized drug delivery was standard practice for children and for emergency treatment of asthma exacerbations, hospitalized patients, and severely obstructed patients. However, nebulized drug delivery is labor intensive; significant cost savings can be realized, without sacrificing efficacy, by using MDIs coupled with spacer devices.
Clinical Use
The β2-Adrenergic agonists remain first-line drugs in the treatment of both asthma and chronic obstructive pulmonary disease (COPD).14,15 In asthma, the short-acting inhaled β2-Adrenergic agonists are preferred for treating acute symptoms and for preventing exercise-induced bronchospasm. The subcutaneous route of administration is generally reserved for patients unresponsive to frequent, high-dose, inhaled β2-Adrenergic agonists; uncooperative patients; or patients too severely dyspneic to inhale the dose. Subcutaneous or parenteral administration should not be used in patients with angina or a recent history of myocardial infarction. Oral adrenergic agonists may be appropriate for children too young to cooperate with inhaled drug administration; sustained-release, oral adrenergic agonists decrease nocturnal symptoms, but they are less effective than LABAs.
In COPD, β2-Adrenergic agonists provide modest symptomatic relief and improvement in pulmonary function. LABAs are standard bronchodilator therapy for patients with moderate and severe COPD. Standard doses of inhaled β2-Adrenergic agonists appear as effective as inhaled anticholinergic drugs for relief of acute exacerbations of COPD. The value of subcutaneous drugs and high-dose, short-acting bronchodilators in the management of COPD remains indeterminate.
The intensity and duration of response to β2-Adrenergic agonists is dose and frequency dependent. For patients with asthma, higher doses result in incrementally greater bronchodilation. The dose–response relationships are less well defined for COPD.
The dose–response curve in asthma led to the development of intensive inhaled β2-Adrenergic agonist drug regimens for the treatment of severe, acute exacerbations. Typically, the nebulized drug is administered every 20 minutes for three to six doses; some patients respond better to continuous nebulized drug delivery. These regimens are generally well tolerated, although cardiac stimulation is common.
The LABAs are add-on agents for patients with moderate or severe asthma when usual doses of inhaled corticosteroids are inadequate and for patients with moderate or severe COPD. The LABAs are also alternate add-on agents for patients with symptoms of nocturnal asthma. The LABAs play no role in the treatment of an acute asthma or COPD exacerbation; all patients should have a short-acting inhaler and should be instructed on how and when to use each type of β2-Adrenergic agonist.
Tolerance, that is, receptor subsensitivity or tachyphylaxis, is a decreased response to receptor stimulation. Regular use of either short- or long-acting β2-Adrenergic agonists leads to tolerance of both the nonbronchodilator and bronchodilator effects of the β2-Adrenergic agonist.16 Tolerance to the nonbronchodilator effects of the β2-Adrenergic agonists, including tremor, tachycardia, QTc prolongation, hypoglycemia, hypokalemia, and vasodilation has been recognized for many years. Tolerance to the bronchodilator effects of the β2-Adrenergic agonists develop rapidly with quick recovery following discontinuation of the drug.17 Receptor downregulation likely mediates tolerance to the β2-Adrenergic agonists.16
Safety
The β2-selective adrenergic agonists produce less cardiovascular toxicity than do the nonselective agents, but β2-selectivity does not protect from all adverse events. Biochemical abnormalities associated with the β2-Adrenergic agonists include hyperglycemia, hyperinsulinemia, lipolysis, hypokalemia, hypomagnesemia, and lactic acidosis.7 These side effects are most pronounced with parenteral and oral drug administration; they are minimal with usual doses of inhaled agents.
β2-Adrenergic agonists cause dose- and route-dependent hyperglycemia by stimulating glycogenolysis and gluconeogenesis and suppressing glucose clearance.18 Hyperglycemia may be clinically most important in asthmatic patients with diabetes mellitus or during pregnancy. β2-Adrenergic agonists increase plasma insulin by directly stimulating pancreatic islet cells; indirect increases occur secondary to the hyperglycemic response. β2-Adrenergic agonists induce the release of free fatty acids from adipose tissue. Although hyperinsulinemia and high concentrations of free fatty acids have been linked with cardiovascular morbidity and mortality, tolerance minimizes these effects. β2-Adrenergic receptor stimulation also induces muscle glycogenolysis, increasing lactate production.
The β2-Adrenergic agonists induce hypokalemia by directly stimulating the uptake of potassium into skeletal muscle cells.19 β2-Adrenergic receptor stimulation induces the cellular uptake of magnesium; hypomagnesemia may induce arrhythmias or worsen symptoms of coronary artery disease. Other adverse β2-Adrenergic agonist effects include (1) an increased baseline tremor by creating an imbalance in fast- and slow-twitch muscle groups; (2) tachycardia by direct chronotropy and through reflex peripheral vasodilatation and decreased venous return; and (3) central nervous symptoms, such as appetite suppression, headache, nausea, and sleep disturbances.7 The nervousness reported by many patients is probably a response to the peripheral tremors, rather than a result of direct stimulation of the central nervous system.
β-Adrenergic agonist use has increased coincident with the increase in asthma morbidity and mortality in the United States and other countries. The first linkage between β-Adrenergic agonists and asthma mortality was identified during the 1960s when the newly marketed, nonselective β-Adrenergic agonist isoproterenol was associated with an increase in asthma morbidity and mortality in the United Kingdom. More recently, LABA use has been associated with an increased risk of death in patients with asthma.20,21 The mechanism is unknown, although it may be related to β-receptor downregulation and desensitization. In 2005, the Food and Drug Administration (FDA) began requiring that labeling for all LABAs include a black box warning regarding the increased risk of asthma-related death. In 2010, the FDA released new safe-use requirements for LABAs. The FDA requires that LABAs (1) be used in combination with an asthma controller medication, and only in patients whose asthma is poorly controlled despite controller medications; (2) be used for the shortest duration of time needed to achieve control and then be discontinued, if possible; and (3) that combination inhaled corticosteroid-LABA products be used for children and adolescents.22 Concurrent corticosteroid use may reduce the risk of death associated with LABAs; FDA-mandated clinical trials designed to answer this question were initiated in 2011.
 ANTICHOLINERGICS
ANTICHOLINERGICS
Atropine, scopolamine, and other naturally occurring antimuscarinic alkaloids from plant extracts have been used for thousands of years to relieve respiratory symptoms in humans with airway diseases. Historically, clinical use of atropine and atropine-like agents has been limited by side effects, including dry mouth and skin, tachycardia, meiosis, and difficulty urinating and mentating. Three synthetic atropine derivatives (ipratropium bromide, tiotropium bromide, and aclidinium bromide), which have more favorable side effect profiles, are available for management of COPD.
Pharmacology
Three of the five known muscarinic receptor subtypes are expressed in the lung (M1, M2, and M3).23 Activation of airway M1 and M3 muscarinic receptors results in bronchial smooth-muscle contraction and mucus secretion. M2 “autoreceptor” activation decreases acetylcholine (Ach) release; blockade of M2 receptors increases airway Ach. The ideal anticholinergic drug would selectively block M1 and M3 receptors and have no effect on M2 receptors.
Ipratropium (FDA approved in 1998), tiotropium (FDA approved in 2004), and aclidinium (FDA approved in 2012) are the bromide salts of a different synthetic quaternary ammonium compound. Each does not penetrate the blood–brain barrier, exhibits minimal systemic absorption, and possesses a longer duration of action than atropine; each possesses differing muscarinic receptor binding affinities and receptor–drug complex half-lives.24
Ipratropium nonselectively blocks M1, M2, and M3 receptors. Tiotropium has greater muscarinic receptor affinity than ipratropium bromide, but it dissociates rapidly from M2 receptors, resulting in prolonged M1– and M3-drug complex half-lives compared with ipratropium. Aclidinium has a kinetic selectivity for M3 receptors, with a longer residence time on the receptor than ipratropium or tiotropium.25
Ipratropium inhalation produces bronchodilation within 15 minutes, with a peak effect after 1 to 2 hours.26 Inhalation of tiotropium produces bronchodilation within 30 minutes, with an effect peak after 3 to 4 hours and duration of action for 24 hours.26 The trough FEV1 increases after multiple doses, reflecting carryover bronchodilation from the prolonged half-life. Inhalation of aclidinium produces maximal bronchodilation at about 2 hours, with sustained bronchodilation for the 12-hours dosing interval.27 Tolerance or tachyphylaxis with chronic use has not been reported.
Drug Delivery
Tiotropium bromide and aclidinium bromide are available in the United States as DPIs (HandiHaler™ and Pressair™, respectively). Ipratropium bromide is available in the United States as a HFA MDI, a solution for nebulization, and as a combination product with albuterol in a soft-mist aerosol inhaler (Combivent Respimat™).
Clinical Use
Ipratropium, tiotropium, and aclidinium are most efficacious in patients with COPD, including emphysema and chronic bronchitis, and they are the bronchodilators of choice in the long-term management of moderate-to-severe COPD.28 In contrast, the role of anticholinergics in the management of asthma has not been established. Compared to the β-Adrenergic agonists, the anticholinergic drugs have a slower onset of action and less effect on lung function.28 The addition of tiotropium to treatment with inhaled corticosteroids and long-acting β-Adrenergic agonists improves symptoms and lung function and decreases the risk of severe exacerbation in selected patients,29,30 but these results cannot be generalized to all patients with asthma.
Safety
Consistent with their very low systemic bioavailability, the inhaled anticholinergics are remarkably free of side effects. Transient or mild dry mouth occurs, but it is rarely a reason for halting therapy.31 Side effects of tiotropium typically appear after 3 to 5 weeks of continued use, reflecting the slow linear tissue accumulation of the drug. Plasma aclidinium is rapidly hydrolyzed to inactive metabolites, reducing systemic exposure to the drug.32
The inhaled anticholinergics do not change mucus viscosity or transport, pulmonary hemodynamics, ventilation–perfusion matching, oxyhemoglobin saturation, heart rate, or urinary flow; however, blurred vision and pupillary dilation may occur if any drug inadvertently contacts the eye. All the three drugs should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia, or bladder neck obstruction. Ipratropium and tiotropium are renally eliminated; patients with moderate or severe renal insufficiency (Clcr <30–50 mL/min) should be monitored closely for systemic anticholinergic effects.33 Less than 0.15% of aclidinium bromide is excreted renally.34
Increased mortality in patients with COPD has been reported with long-term (≥30 days) use of tiotropium delivered with the fine-mist Respimat™ inhaler.35 However, a major recent trial directly comparing tiotropium dry powder and fine-mist inhaler safety revealed no significant differences in either safety or efficacy.36 Until the risks associated with the fine-mist inhaler are fully described, patients with COPD, patients with hypoxia, cardiac arrhythmias, or ischemic heart disease should use the dry powder form of tiotropium.37
Concomitant use of other drugs with anticholinergic properties may increase the risk of side effects with either ipratropium or tiotropium.
 METHYLXANTHINES
METHYLXANTHINES
Methylxanthines have been used to treat pulmonary disease for more than 100 years.38 General use of methylxanthines became widespread in the 1930s; contemporary use is more limited due to the availability of more effective and safer medications.39 Theophylline and aminophylline, the ethylenediamine salt of theophylline, are used to treat asthma and COPD. Theophylline may also have a role in therapy of obstructive sleep apnea, apnea of prematurity, and airway obstruction secondary to pulmonary edema.
Pharmacology
Potentially beneficial therapeutic effects of theophylline include bronchial smooth-muscle relaxation, enhanced mucociliary transport, inhibition of mediator release, suppression of permeability edema, decreased pulmonary hypertension, increased right ventricular ejection fraction, improved diaphragmatic contractility, and central stimulation of ventilation.
Methylxanthines have direct bronchodilator and immunomodulatory properties; however, despite having been marketed and studied for decades, the precise molecular mechanism of action is not known. Multiple molecular mechanisms have been proposed, including nonselective phosphodiesterase (PDE) inhibition, nonselective adenosine receptor antagonism (A1, A2A, A2B, and A3 receptors), promotion of endogenous catecholamine release, agonist activity at ryanodine receptors, activation of histone deacetylase (HDAC), and induction of peroxisome proliferator-activated receptor gamma (PPAR gamma) expression.39,40 Nonselective PDE inhibition and adenosine receptor antagonism are the most established of the proposed molecular mechanisms of action.39
PDEs hydrolyze cAMP and cyclic guanine monophosphate (cGMP) to inactive 5′ monophosphates; inhibition of PDE increases intracellular concentrations and, therefore, the activity of these secondary messengers, resulting in airway smooth-muscle relaxation and bronchodilation. However, methylxanthine PDE inhibition is concentration dependent, with minimum inhibition seen at clinically relevant serum concentrations.41 Adenosine is a nucleoside signaling molecule. Adenosine receptor antagonism regulates airway smooth muscle, blocks adenosine-induced mast cell degranulation, and activates HDAC enzymatic activity, inhibiting transcription of proinflammatory cytokines. These anti-inflammatory and immunomodulatory effects are observed at clinically achievable serum drug concentrations.
The anti-inflammatory effect of methylxanthines appears to be qualitatively different than that of corticosteroids. Corticosteroids induce HDAC gene transcription; methylxanthines induce HDAC activity. The combination of corticosteroids and methylxanthines may be synergistic.
Structure–Activity Relationships
Theophylline and aminophylline are 1,3-dimethylxanthines. Other methylxanthines, including theobromine (3,7-dimethylxanthine) and caffeine (1,3,7-trimethylxanthine), differ in the positions of the methyl substituents on the xanthine molecule. Dyphylline is 7-(2,3-dihydroxypropyl)-theophylline. N-1 substituents are important for adenosine antagonism, whereas N-3 substituents augment bronchodilator activity. Substituents at N-7 decrease bronchodilator potency; substituents at N-9 decrease the potency of the xanthine.
Drug Delivery
Three methylxanthines are available in the United States: theophylline, aminophylline, and dyphylline. Dosage forms for theophylline include extended-release tablets and capsules, oral solution, oral elixir, and intravenous solution. Aminophylline, the ethylenediamine salt of theophylline, is only available as an intravenous solution. Dyphylline is available as immediate-release tablets.
Clinical Use
Methylxanthines are FDA approved for the treatment of reversible airway obstruction associated with chronic asthma or other chronic lung disease. Concerns regarding the relative efficacy and safety of the methylxanthines in comparison to other bronchodilators and long-term control medications have relegated methylxanthines to alternative therapy status for both asthma and COPD.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend oral theophylline as an alternative maintenance treatment choice for all patients with COPD or as combination therapy with any first- or second-line treatment.15 Intravenous theophylline or aminophylline are second-line choices for treatment of exacerbations of COPD.15 The current Global Initiative for Asthma guidelines recommend theophylline as an add-on controller medication for patients inadequately controlled on inhaled glucocorticoids alone.42 The current National Asthma Education and Prevention Program (NAEPP) guidelines recommend theophylline as an alternate medication for patients with mild persistent asthma, or as an alternate adjunctive therapy in combination with inhaled corticosteroids.14 The NAEPP guidelines do not recommended theophylline for the treatment of acute asthma exacerbations.14
In summary, relatively noncontroversial indications for methylxanthines include severe bronchodilator-dependent COPD; severe, systemic, corticosteroid-dependent asthma; nocturnal asthma uncontrolled with adrenergic agonists; and acute, severe asthma progressing to respiratory failure.
The previously accepted therapeutic range for theophylline (10–20 mg/L) was based on early dose–bronchodilator response studies43 and is no longer considered state of the art.44 The current NAEPP asthma guidelines recommend a lower target therapeutic range for theophylline (5–15 mg/L).14 The rationale for lowering the target serum theophylline range includes improved understanding of the dose–bronchodilator response and recognition of the greater risk of adverse drug reactions at serum theophylline concentrations greater than 15 mg/L.45 The immunomodulatory effects of theophylline occur at lower serum concentrations (1–5 mg/L).46,47
Safety
Adverse effects of methylxanthines are generally attributed to PDE inhibition (nausea, vomiting, diarrhea, headache) and adenosine receptor antagonism (cardiac arrhythmias and seizures).47 Other adverse effects include irritability and insomnia. The caffeine-like adverse effects (nausea, vomiting, diarrhea, nervousness, insomnia, headache) are generally associated with serum theophylline concentrations greater than 15 mg/L.45 The more life-threatening adverse effects (ventricular arrhythmias and seizures) are generally associated with serum theophylline concentrations greater than 40 mg/L, although these adverse effects may occur at much lower serum concentrations.45
Theophylline is metabolized primarily by cytochrome P450 (CYP1A2) enzymes, making it susceptible to numerous drug–disease and drug–drug interactions. Medical conditions that directly impair hepatic function (e.g., cirrhosis, viral hepatitis), cause passive hepatic congestion (e.g., congestive heart failure, cor pulmonale), or induce interferon (e.g., acute viral illness or pneumonia) increase the serum theophylline concentrations.45
Hyperthyroidism and cystic fibrosis decrease serum theophylline concentrations by unknown mechanisms. Polycyclic aromatic hydrocarbons in tobacco smoke, and medications, such as rifampin, phenytoin, antivirals, and barbiturates, induce cytochrome P450 enzymes, reducing serum theophylline concentrations. Macrolide and quinolone antibacterials, fluvoxamine, fluconazole, ketoconazole, and cimetidine inhibit cytochrome P450 enzymes, increasing serum theophylline concentrations. Given the narrow therapeutic window, multitude of drug and disease interactions, and wide interpatient variability, it is important to routinely monitor serum theophylline concentrations. The mid-dosing interval serum concentration should be assessed 3 to 5 days after initiating theophylline or modifying the dose and any time theophylline toxicity is suspected.14
 OTHER BRONCHODILATORS
OTHER BRONCHODILATORS
Ancillary drugs sometimes used as bronchodilators include magnesium sulfate and inhaled diuretics.
Magnesium Sulfate
The bronchodilatory mechanism of action of magnesium has not been definitively identified. Several actions have been proposed, including decreasing smooth-muscle intracellular calcium, T-cell stabilization, inhibition of mast cell degranulation and Ach release, and stimulation of nitric oxide and prostacyclin synthesis.48
Several systematic reviews of trials of inhaled and intravenous magnesium sulfate in the management of adults and children presenting to emergency departments with severe acute exacerbations of asthma have been published.48–50 In general, patients with severe exacerbations demonstrate small improvements in pulmonary function following administration of inhaled or intravenous magnesium sulfate. Potential adverse effects include hypotension with rapid infusion, muscle weakness, respiratory depression, and cardiac conduction abnormalities. Despite these limited clinical data, the Global Initiative for Asthma recommends consideration of intravenous magnesium sulfate therapy for acute severe asthma in selected patients (adults with an FEV1 25%–30% of predicted at presentation, adults and children who do not respond to initial treatment, and children in whom the FEV1 remains <60% of predicted after 1 hour of standard treatment).42
Inhaled Diuretics
Inhaled diuretics (e.g., furosemide) may act upon the airways through a variety of mechanisms to achieve bronchodilation, reduce mucosal inflammation, or interrupt sensory nerve reflex responses to irritants.51 Data regarding the clinical efficacy of inhaled diuretics in the treatment of acute and chronic asthma are limited. Additional information from well-designed clinical trials is needed before use of inhaled diuretics can be recommended for the treatment of acute exacerbations or chronic management of asthma.
ANTI-INFLAMMATORY AGENTS
Corticosteroids are the mainstay of current anti-inflammatory regimens; other agents in clinical use include mast cell stabilizers, leukotriene receptor antagonists, and leukotriene formation inhibitors.
 CORTICOSTEROIDS
CORTICOSTEROIDS
Corticosteroids are cortisol-like drugs that influence metabolic pathways and have an anti-inflammatory effect. By reducing airway inflammation, corticosteroids have long been known to be useful in the management of asthma; their role in the management of COPD has been increasingly recognized.
Pharmacology
Cortisol is produced by the adrenal cortex and regulated via the hypothalamic–pituitary axis in response to physical and emotional distress. Although the usual daily secretion of cortisol is approximately 10 mg/m2/d,52 up to 500 mg/d can be secreted during periods of severe stress.53 Corticosteroids stimulate and inhibit DNA transcription in cells with glucocorticoid receptors, which are widespread throughout the body.
Glucocorticoids diffuse across cellular membranes to bind inactive glucocorticoid cytoplasmic receptors; these activated glucocorticoid-receptor complexes translocate to the nucleus and bind to specific glucocorticoid response elements of the gene, influencing gene transcription. Increased gene transcription increases expression of β2-Adrenergic receptors, increases the production of anti-inflammatory mediators, and decreases the production of inflammatory mediators.54 The clinical effects of glucocorticosteroids are delayed for several hours following administration, reflecting the time needed to influence gene transcription. In the airways, glucocorticosteroids increase the number and responsiveness of β2-Adrenergic receptors, decrease mucus production, decrease bronchial hyperresponsiveness, decrease the number of mucosal mast cells, enhance eosinophil apoptosis, and decrease airway edema.55 Glucocorticosteroids are inactivated by hepatic metabolism, with a clearance rate approximately the same as liver blood flow; beclomethasone dipropionate and ciclesonide and their metabolites are also inactivated by blood esterases.
The inhaled glucocorticoids (beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone propionate, mometasone furoate) have different physicochemical properties (potency, receptor-binding affinity, protein binding, lipophilicity, oral bioavailability, pulmonary bioavailability, systemic clearance, volume of distribution, and half-life).56,57 Physicochemical properties that enhance inhaled glucocorticosteroid efficacy include high receptor-binding affinity, high pulmonary bioavailability, and long pulmonary retention time. Physicochemical properties that enhance safety include low oropharyngeal deposition, high protein binding, rapid systemic clearance, and conversion of inactive prodrug to active drug in the lungs. Since inhaled glucocorticosteroids are marketed and prescribed in relatively equipotent doses, potency is not an important differentiating characteristic as other physicochemical characteristics.
Data regarding the relative clinical benefits of inhaled corticosteroids with different physicochemical characteristics are limited. However, physicochemical characteristics may influence drug safety. For example, oropharyngeal side effects (dysphonia, oral candidiasis) may be minimized by using drug delivery systems that generate a greater proportion of small particles (<5 μm)58 and with administration of prodrugs that are converted in the lungs to the active drug. DPI particle size depends on the specific device and the patient-generated inhalation flow rate; hydrofluoroalkane MDIs tend to generate a greater proportion of smaller particles. Beclomethasone 17,21-dipropionate is hydrolyzed in the lungs to the more active drug, beclomethasone 17-monopropionate. Ciclesonide is hydrolyzed in the lungs from the inactive parent drug to the active drug, desiosbutyryl-cilcesonide.
The risk of systemic adverse effects may be minimized by using inhaled corticosteroids with low systemic bioavailability, high protein binding, rapid systemic clearance, and increased pulmonary residence time. Ciclesonide, fluticasone, and mometasone undergo rapid first pass hepatic metabolism, resulting in very low (<1%) oral bioavailability. Ciclesonide and mometasone are highly protein bound (99%). Drugs with a higher lipophilicity and drugs that undergo lipid conjugation have a longer pulmonary residence time. Mometasone furoate and fluticasone propionate, with an esterified lipophilic group at the 17-α position, are the most lipophilic of the marketed inhaled corticosteroids; beclomethasone dipropionate, budesonide, triamcinolone acetonide, and flunisolide follow in descending order of lipophilicity.
Clinical Use
Corticosteroids are the cornerstone of therapy in the treatment of asthma. The role of glucocorticoid therapy in COPD is more limited.
Asthma The role of airway inflammation in asthma is well established, and high-dose systemic (parenteral or oral) corticosteroids are standard therapy for patients experiencing severe acute exacerbations of asthma.14,42 Parenteral administration of corticosteroids is often used preferentially due to the inability of some patients to swallow medications while in respiratory distress or because of lack of oral access after intubation. The time to initial response, as evidenced by augmentation of FEV1 with bronchodilator administration, begins as early as 1 hour after corticosteroid administration; maximal response is achieved in 8 to 12 hours. Parenteral methylprednisolone is the corticosteroid of choice, due to its lower mineralocorticoid and greater glucocorticoid effects than hydrocortisone.
The combination of an inhaled corticosteroid and a long-acting bronchodilator is better than either agent alone at improving lung function and preventing asthma exacerbations; in patients with moderate-to-severe asthma, such a combination of low-dose inhaled corticosteroid with a LABA appears equieffective as high-dose inhaled corticosteroid alone.14 Given the concerns regarding the safety of LABAs (see above) as monotherapy in asthma,20 the FDA recommends that an inhaled corticosteroid always accompany a LABA in asthma therapy.22
Beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, and fluticasone propionate are FDA approved for twice-daily administration; only mometasone furoate is FDA approved for once-daily administration. Poor adherence with inhaled corticosteroid regimens is an ongoing issue. Although data are limited, patients with stable, mild persistent asthma might benefit from once-daily inhaled corticosteroids.14 Twice-daily dosage regimens are more effective for patients with moderate persistent or severe persistent asthma.14
Chronic Obstructive Pulmonary Disease The mechanism underlying the beneficial effects of corticosteroids in COPD is not fully known, but changes in inflammatory gene transcription and modulation of β2-Adrenergic receptor function appear to play a role. For patients with COPD with an FEV1 <60% (GOLD grades C and D), treatment with inhaled corticosteroids improves quality of life and lung function, decreases exacerbation frequency, but does not alter long-term FEV1 decline or improve survival.15 Short courses (5–15 days) of moderate doses (40 mg/d of prednisone) of systemic corticosteroids in combination with standard bronchodilator therapies are effective for treating acute exacerbations of COPD in outpatients, inpatients, and patients in the emergency room who have moderate or severe COPD.59 However, the role of systemic corticosteroids in the treatment of patients with COPD who are receiving mechanical ventilation is unknown. Definitive data regarding the clinical usefulness of combining inhaled corticosteroids and long-acting bronchodilators are lacking.15
Safety
Short-term use (less than 14 days) of systemic corticosteroids is associated with mild glucose intolerance, fluid retention that may progress to edema and hypertension, proximal muscle weakness (especially with large parenteral doses), and mood alteration. Long-term systemic corticosteroids prolong the short-term effects; in addition, peptic ulcer disease, cataracts, increased risk of infection, and impaired wound healing occur. Truncal obesity, hirsutism, acne, moon-shaped facies, striae, and ecchymoses contribute to a cushingoid appearance. Disruption of bone metabolism predisposes patients to osteoporosis and resultant vertebral and long-bone fractures; inhibition of long-bone growth is the major complication in children receiving systemic corticosteroids. Suppression of the hypothalamic–pituitary–adrenal axis diminishes body cortisol stores, reducing the response to stresses such as trauma, surgery, or infection.
Inhaled corticosteroids are less systemically bioavailable due to poor absorption from the tracheobronchial tree. The most common adverse effect is local irritation of the oropharynx, cough, and bronchospasm. Dysphonia may arise from vocal cord myopathy induced by the corticosteroid, and oral candidiasis (thrush) may occur secondary to suppression of normal oral flora. The risk of dysphonia and thrush are reduced by rinsing the mouth, gargling, and spitting out the rinse water after each use of a corticosteroid inhaler; using a spacer device to decrease deposition of drug particles in the mouth; and keeping the inhaler mouthpiece clean. Limited data suggest inhaled corticosteroids increase the risk of pneumonia in patients with COPD,60 but not asthma.61
Depending on the bioavailability, dose, and duration of use, inhaled corticosteroids may be associated with the systemic adverse effects typically associated with oral corticosteroids, including adrenal suppression, particularly in children. Inhaled corticosteroids slow bone growth velocity in prepubertal children and reduce ultimate adult height62; they also increase the risk of bone fracture, with each 500 μg increase in beclomethasone equivalency yielding a 9% increase in fracture risk.63
Steroid Resistance
Patients with asthma unresponsive to usually sufficient doses of corticosteroids are described as steroid resistant. Varying definitions of steroid resistance exist, such as a <15% increase in FEV1 after either oral prednisolone administered for 7 days at a dose of 20 mg daily, or 14 days at a dose of 15 mg daily; or a 2-week trial of 40 mg/d prednisone in bronchodilator-responsive asthmatics.64,65 Steroid resistance must be distinguished from steroid dependency, which is usually defined as the need for systemic corticosteroids to maintain control of asthma.
Steroid resistance may involve reduced metabolism of oral corticosteroids to the active compound or accelerated drug clearance. An impaired cellular response to corticosteroids has been observed in steroid-resistant asthmatics, and altered receptor binding or the presence of anti-lipocortin antibodies may contribute to the phenomenon.
 CORTICOSTEROID-SPARING AGENTS
CORTICOSTEROID-SPARING AGENTS
Chronic systemic corticosteroid therapy required for the treatment of severe airflow obstruction often results in numerous side effects. Therefore, many anti-inflammatory agents have been evaluated in an effort to identify alternatives to systemic corticosteroid therapy.66,67 However, wide variability exists between studies in their assurances of proper inhaler technique, dose and frequency of baseline inhaler regimens, and minimization of exposure to known antigens. In addition, most studies did not systematically evaluate and exclude (or otherwise control for) the concomitant presence of disorders known to mimic or exacerbate asthma, including vocal cord dysfunction, gastroesophageal reflux, and rhinosinusitis. These methodological considerations further restrict the limited conclusions to be drawn from these studies.
Methotrexate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


