Pulmonary Paragonimiasis and Its Surgical Complications
Hiroshi Mukae
Fukumi Nakamura-Uchiyama
Yufigumi Nawa
Paragonimiasis is a subacute to chronic inflammatory disease of the lung caused by lung flukes of the genus Paragonimus. It is a food-borne parasitic disease common in East Asia, particularly in Japan, Korea, China, Taiwan, and the Philippines. A high incidence of paragonimiasis is also observed in some parts of Africa and Latin America. It rarely occurs naturally in the United States, and patients identified in North America have mostly been emigrants or refugees from endemic areas, as reported by Johnson and Johnson9 and Taylor and Swett.27 Blair and coworkers2 noted that among the >40 species of Paragonimus described to date, Paragonimus westermani is the most frequent etiologic agent, which infects millions of people throughout Asia. Several other species, including P. miyazakii, P. uterobilateralis, P. africanus, P. mexicanus, and P. kellicotti, also cause pulmonary disease. Paragonimiasis is closely related to eating habits. Infection results from ingestion of raw or inadequately cooked freshwater crabs and crayfish, which are intermediate hosts for the organism. According to Miyazaki and Hirose,15 raw flesh of crab-eating mammals like wild boars can also be a source of the paratenic human infection. This mode of infection appears to be increasing in Japan, as reported by two of us (F.N.U. and Y.N.) and a colleague.31 Paragonimiasis is rarely seen in Europe and North America, but one patient, reported by Guiard-Schmid and associates,6 appeared to be infected while visiting Japan. Meehan and colleagues14 also reported that a patient with paragonimiasis who had resided in the north central United States for 8 years, habitually ate pickled or frozen crabmeat imported from Southeast Asia. These findings suggest that the disease will be encountered more frequently in various parts of the world in the future owing to the popularization of Asian foods and increasing international travel. Of note is that P. kellicotti exists in parts of the midwestern and eastern United States, as reported by Ameel.1 However, human infection in the United States is rare because the ingestion of raw freshwater crab or crayfish does not commonly occur.
However, recently DeFrain and Hooker4 reported a case of paragonimiasis that developed in an 18-year-old man after the ingestion of raw freshwater crayfish during a camping trip in northern Michigan. Procop and associates21 also reported a case in a patient with no history of foreign travel or ingestion of foreign foods.
Etiology
Life Cycle of Paragonimus
Paragonimus is a highly adapted organism that reproduces through a complex life cycle. The definitive host harbors the adult worm in the lung, where it produces eggs that are voided from the host in sputum. Eggs are frequently swallowed and often present in the feces. Under suitable conditions, eggs lying in moist soil or water hatch as miracidiae, which infest freshwater snails, the first intermediate host. In the snail, miracidiae develop into cercariae, which subsequently invade freshwater crabs or crayfish, the second intermediate hosts. There they develop into metacercariae, an infective form. Infection of the final host occurs by eating the flesh of raw crayfish or freshwater crabs contaminated with the metacercariae. Handling the raw flesh or juice may also result in transfer of metacercariae from the hand into the mouth. When freshwater crabs or crayfish contaminated with the metacercariae are ingested by unsuitable mammalian hosts, such as wild pigs and boars, juvenile worms excysted from metacercariae migrate into the muscles of the hosts, where they can remain in an immature state for many years, as reported by Miyazaki and Hirose.15
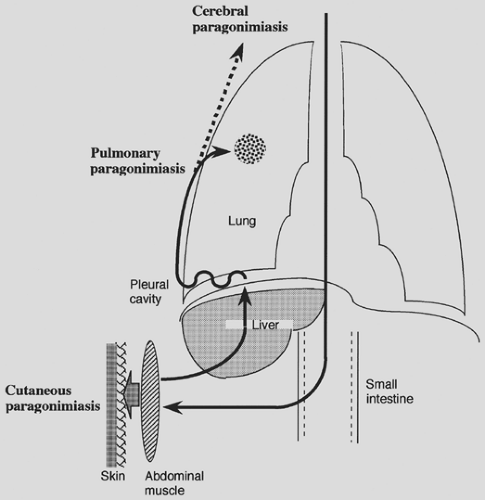
Migration in the Definitive Host
A typical route of migration of P. westermani in humans or other natural final hosts is as follows (Fig. 95-1). When metacercariae are ingested by the final host, they excyst in the intestine, penetrate into the abdominal cavity, and then reside in the abdominal muscles for about a week. Then the larvae migrate through the peritoneum, liver, and diaphragm and into the pleural space. Here they penetrate the lungs, where they finally become mature adult flukes. This migration process takes several weeks. They commence laying eggs in the lungs and can live there for many years. The life cycle of the parasite is complete when eggs are returned to the environment to repeat the process. On some occasions the worms accidentally migrate into other sites, such as subcutaneous tissue, liver, and brain (extrapulmonary para-gonimiasis).
Clinical Features
Clinically, symptoms related to paragonimiasis parallel the migration and maturation of the parasite. According to Tokojima and colleagues,28 patients with pulmonary paragonimiasis exhibit primarily respiratory symptoms, including cough (70%), hemoptysis (reddish brown–colored sputum, 35%), chest pain (35%), and dyspnea (17%). Other symptoms—such as low-grade fever (22%), subcutaneous tumors (9%), diarrhea (4%), abdominal pain (4%), and weight loss (4%)—are also reported. Although clinical findings depend on the worm load, patients usually maintain a better state of general health than what is predicted from their chest radiographs. Few signs are observed on physical examination, although some patients can have audible crepitations in the chest. In cases of cutaneous paragonimiasis, a slow-moving, nodular lesion in the subcutaneous tissue of the abdominal or anterior chest wall is characteristic. Paragonimiasis in the brain can be fatal and can cause epileptic seizures, hemiplegia, and encephalitis. For clinical diagnosis, the patient’s history, including eating habits, is usually more important than the physical examination.
Peripheral blood eosinophilia and elevated serum immunoglobulin E (IgE) are observed in patients with paragonimiasis, as in other helminth parasite infections, but the total white blood cell count usually remains in the normal range or is only slightly elevated. Two of us (FNU and YN) and an associate19 recently reported that the degree of peripheral blood eosinophilia was slightly higher in patients with pleurisy compared with patients who have intrapulmonary lesions. Tokojima and coworkers28 reported that peripheral blood eosinophilia was seen in only 56.5% of patients with pulmonary paragonimiasis. Thus, at least some patients, especially those with only parenchymatous lesions, may have a normal peripheral eosinophil count. Lack of eosinophilia, therefore, should not be used to exclude the possibility of Paragonimus infection. However, a marked eosinophilia is commonly seen in samples from affected lesions like pleural effusion and bronchoalveolar lavage fluid (BALF), as reported previously by one of us (HM) and associates.16 Two of us (FNU and YN) and a colleague19 noted that the total serum IgE level, which is also an important parameter for parasitic diseases, has no correlation with the stage of paragonimiasis. Other laboratory tests appear to be of little diagnostic value.
Paragonimiasis can produce a range of different radiographic images. We (FNU, HM, and YN)18 noted intrapulmonary lesions in paragonimiasis can be roughly classified as nodular (Fig. 95-2A,B,C), infiltrative (Figs. 95-2D and 95-3A), cavitating (Fig. 95-3B), or a combination. Ogakwu and Nwokolo20 have described four patterns: the most common were well-defined shadows or patches of cavitation, ill-defined “cotton wool” lesions, “streaky” shadows, or “bubble” cavities. These
patterns were identified predominantly in the midzones of the lungs. In this study, normal chest radiographs were also observed in approximately 20% of patients in whom Paragonimus eggs were identified. However, a somewhat different radiologic presentation was observed by Johnson and Johnson,9 who described diffuse (44%) and segmental (24%) infiltrates, nodules (20%), and cavities (20%). Cavitating lesions on chest radiographs are sometimes described as “ring shadows,” which were the most prominent lesion in a study of 38 cases in Thailand by Suwanik and Harinsuta.25 Im and colleagues7 emphasized that airspace consolidation with or without cystic changes was the most common findings in Korea. In a recent experience in Japan, one of us (HM) and colleagues16 found that the most common lesion was a nodular shadow (see Fig. 95-2), a pattern distinct from those observed in the aforementioned studies. In addition, chest computed tomography
(CT) scans revealed that the nodules were frequently located near bronchioles or bronchi, predominantly in the subpleural region and occasionally accompanied by ectatic changes in associated bronchi. Atelectasis is observed less frequently because of compression by the massive pleural effusion or bronchial stenosis. Mediastinal lymphadenopathy is also occasionally seen. On chest radiography, the differential diagnosis of pulmonary paragonimiasis should therefore include lung cancer, tuberculosis, and fungal infection. In this context, Johnson and Johnson9 noted that 68% of their patients with paragonimiasis had been treated erroneously for pulmonary tuberculosis. Yoshino and colleagues34 reported on a patient in whom, following normal routine laboratory examination results and the absence of any clinical symptoms, P. westermani infection was diagnosed after an open lung biopsy was performed for a suspected lung cancer. Tomita and associates30 likewise reported six patients who were referred to the department of surgery because they had mass lesions on chest radiography that were indistinguishable from malignancy. Pleural lesions such as pleural effusion or pneumothorax are also often observed in infected patients. Johnson and Johnson9 noted that 48% of patients had pleural effusions. We (FNU)19 and (HM)16 and our coworkers have likewise noted that nearly 70% of patients in Japan with paragonimiasis present with pleural lesions. Similarly, 61% of Korean patients develop pleural lesions in their clinical course, as reported by Im and colleagues.7 Because Paragonimus worms pass through the pleural cavity before they reach the lungs, a high incidence of pleural lesions is not surprising. One of us (HM) and associates16 have previously reported that some patients showed typical clinical features of paragonimiasis with transient pleurisy followed by parenchymal lesions several months later, which was consistent with the migratory route.
patterns were identified predominantly in the midzones of the lungs. In this study, normal chest radiographs were also observed in approximately 20% of patients in whom Paragonimus eggs were identified. However, a somewhat different radiologic presentation was observed by Johnson and Johnson,9 who described diffuse (44%) and segmental (24%) infiltrates, nodules (20%), and cavities (20%). Cavitating lesions on chest radiographs are sometimes described as “ring shadows,” which were the most prominent lesion in a study of 38 cases in Thailand by Suwanik and Harinsuta.25 Im and colleagues7 emphasized that airspace consolidation with or without cystic changes was the most common findings in Korea. In a recent experience in Japan, one of us (HM) and colleagues16 found that the most common lesion was a nodular shadow (see Fig. 95-2), a pattern distinct from those observed in the aforementioned studies. In addition, chest computed tomography
(CT) scans revealed that the nodules were frequently located near bronchioles or bronchi, predominantly in the subpleural region and occasionally accompanied by ectatic changes in associated bronchi. Atelectasis is observed less frequently because of compression by the massive pleural effusion or bronchial stenosis. Mediastinal lymphadenopathy is also occasionally seen. On chest radiography, the differential diagnosis of pulmonary paragonimiasis should therefore include lung cancer, tuberculosis, and fungal infection. In this context, Johnson and Johnson9 noted that 68% of their patients with paragonimiasis had been treated erroneously for pulmonary tuberculosis. Yoshino and colleagues34 reported on a patient in whom, following normal routine laboratory examination results and the absence of any clinical symptoms, P. westermani infection was diagnosed after an open lung biopsy was performed for a suspected lung cancer. Tomita and associates30 likewise reported six patients who were referred to the department of surgery because they had mass lesions on chest radiography that were indistinguishable from malignancy. Pleural lesions such as pleural effusion or pneumothorax are also often observed in infected patients. Johnson and Johnson9 noted that 48% of patients had pleural effusions. We (FNU)19 and (HM)16 and our coworkers have likewise noted that nearly 70% of patients in Japan with paragonimiasis present with pleural lesions. Similarly, 61% of Korean patients develop pleural lesions in their clinical course, as reported by Im and colleagues.7 Because Paragonimus worms pass through the pleural cavity before they reach the lungs, a high incidence of pleural lesions is not surprising. One of us (HM) and associates16 have previously reported that some patients showed typical clinical features of paragonimiasis with transient pleurisy followed by parenchymal lesions several months later, which was consistent with the migratory route.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree