After reading this chapter you will be able to: Infection involving the lungs is termed pneumonia or lower respiratory tract infection and is a common clinical problem in the practice of respiratory care. In the late 1800s, Osler remarked that pneumonia is “captain of the men of death” because of its poor prognosis in the preantibiotic era. More than a century later, pneumonia remains a major cause of morbidity and mortality in the United States and around the world. Each year, 5 million people die from pneumonia worldwide. In the United States, it is estimated that 5 million cases of pneumonia occur annually, of which approximately 1 million require hospitalization, at a projected yearly cost of more than $20 billion.1 Pneumonia is the seventh leading cause of death in the United States and the most common cause of infection-related mortality.2 Pneumonia can be classified based on the clinical setting in which it occurs (Table 22-1). This classification is useful because it predicts the likely microbial causes and determines empiric antimicrobial chemotherapy while a definitive microbiologic diagnosis is awaited. (The term empiric therapy refers to treatment that is initiated based on the most likely cause of infection when the specific causative organism is still unknown.) TABLE 22-1 Classifications and Possible Causes of Pneumonia Pneumonia acquired in health care settings is often caused by different microorganisms than community-acquired pneumonia. Previously termed nosocomial pneumonia, this clinical entity has been further classified as health care–associated pneumonia (HCAP), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP).3 HCAP is defined as pneumonia occurring in any patient hospitalized for 2 or more days in the past 90 days in an acute care setting or who in the past 30 days has resided in a long-term care or nursing facility; attended a hospital or hemodialysis clinic; or received intravenous antibiotics, chemotherapy, or wound care. HAP is defined as lower respiratory tract infection that develops in hospitalized patients more than 48 hours after admission and excludes community-acquired infections that are incubating at the time of admission. VAP is defined as lower respiratory tract infection that develops more than 48 to 72 hours after endotracheal intubation. HAP is a common clinical problem and represents the second most common nosocomial infection in the United States, accounting for 15% to 18% of all such infections.4,5 Current estimates suggest that more than 250,000 individuals develop this complication each year. HAP increases hospital length of stay 7 to 9 days at an average incremental per-patient cost of $40,000. In selected patient populations, such as patients in the intensive care unit (ICU) and bone marrow transplant recipients, the crude mortality rate from HAP may approach 30% to 70%, with attributable mortality of 33% to 50%. Certain microorganisms, such as Pseudomonas aeruginosa and Acinetobacter species, are associated with higher rates of mortality.6 Six pathogenetic mechanisms may contribute to the development of pneumonia (Table 22-2). To minimize nosocomial spread, knowledge of these mechanisms is important to the understanding of the various disease processes and to the formulation of effective infection control strategies within the hospital. The fact that tuberculosis is acquired by inhalation of infectious particles is the basis for a policy whereby patients with suspected or proven tuberculosis who are coughing are placed in respiratory isolation, minimizing the risk of disease transmission within the hospital setting. TABLE 22-2 Pathogenetic Mechanisms Responsible for the Development of Pneumonia Aspiration seems to be the major mechanism responsible for the development of some types of mixed aerobic and anaerobic, gram-negative, and staphylococcal HAP. In intubated patients, chronic aspiration of colonized secretions through a tracheal cuff has been linked to the subsequent occurrence of pneumonia,4 which has led to the development of novel strategies to prevent HAP, such as continuous suctioning of subglottic secretions in mechanically ventilated patients and elevation of the head of the bed.7,8 Pneumonia also may develop when a latent infection, acquired earlier in life, is reactivated. This reactivation may occur for no apparent reason, as in the case of reactivation pulmonary tuberculosis, but most often it is attributable to the development of cellular immunodeficiency. Pneumocystis jiroveci (previously called Pneumocystis carinii) pneumonia is a prime example of lower respiratory tract infection arising as a result of this mechanism. In developed countries, most healthy individuals have acquired P. jiroveci by age 3 years and show serologic evidence of prior infection. The organism remains dormant in the lung but may reactivate later in life and produce pneumonia in individuals with compromised cell-mediated immunity, such as patients with human immunodeficiency virus (HIV) infection or recipients of long-term immunosuppressive therapy. Cytomegalovirus pneumonia is another example of a latent infection that can reactivate during chronic immunosuppression, especially in solid organ and bone marrow transplant recipients. Immunosuppressive drugs used to modify inflammatory diseases, such as tumor necrosis factor inhibitors, have been associated with pulmonary and extrapulmonary tuberculosis.9 In most studies, S. pneumoniae, also called pneumococcus, has been the most commonly identified cause of community-acquired pneumonia, accounting for 20% to 75% of cases (Table 22-3). Various other organisms have been implicated with varying frequencies. H. influenzae, Staphylococcus aureus, and gram-negative bacilli each account for 3% to 10% of isolates in many reports.10 Legionella species, Chlamydophila pneumoniae, and Mycoplasma pneumoniae together account for 10% to 20% of cases. These latter organisms, called atypical pathogens, vary in frequency in more recent reports, depending on the age of the patient population, the season of the year, and geographic locale. Legionellosis and C. pneumoniae, in particular, seem to exhibit significant geographic variation in incidence. TABLE 22-3 Frequency of Pathogens in Community-Acquired Pneumonia Many studies examining the epidemiology and microbiology of community-acquired pneumonia are potentially biased because they focus on patients requiring hospitalization. In patients with less severe illnesses not requiring hospitalization, more recent studies suggest that organisms such as M. pneumoniae and C. pneumoniae account for 38% of cases and may be more common than typical bacterial pathogens such as pneumococcus and H. influenzae.11 In patients who are ill enough to require admission to the ICU, Legionella species, gram-negative bacilli, and pneumococcus are disproportionately more common.1 A virulent strain of methicillin-resistant S. aureus (MRSA) has emerged as a cause of severe necrotizing community-acquired pneumonia.12 In urban settings that have a high incidence of endemic HIV infection, P. jiroveci may be a more common cause of community-acquired pneumonia and, according to one report, may account for 13% of cases.13 Viruses such as influenza, respiratory syncytial virus, and adenovirus are occasional causes of community-acquired pneumonia, especially in patients with milder illnesses not requiring hospitalization and encountered in the late fall and winter months. A worldwide pandemic of H1N1 influenza during 2009-201014 and ongoing sporadic cases of transmission of H5N1 influenza from birds to humans have led to heightened international awareness of influenza epidemiology, pathogenesis, and prevention. The outbreak in 2000-2001 of inhalation anthrax in the United States adds another microbial differential diagnostic consideration in patients with fulminant community-acquired lower respiratory tract infection.15 To date, inhalation anthrax remains a rare disease. However, it must be considered in selected clinical and epidemiologic settings (see later). A new human pathogen, severe acute respiratory syndrome–associated coronavirus, emerged and spread worldwide in 2002-2003. No cases have been identified since 2004, but this virus should also be considered in the appropriate clinical and epidemiologic setting.16 • Inability of many patients to produce sputum • Failure to perform numerous serologic studies routinely in all patients • The fact that many organisms (e.g., viruses and anaerobic bacteria) were not routinely sought • Failure, until more recently, to recognize “new” pneumonia pathogens, such as C. pneumoniae The common microbial agents producing HCAP, HAP, and VAP are summarized in Table 22-1 and include gram-negative bacilli, S. aureus, Legionella species, and, rarely, viruses such as influenza or respiratory syncytial virus. The last-mentioned viruses are considerations only during the winter months, when they are endemic in the community and may be brought into the hospital by health care workers, visitors, or patients with incubating or active infections. In the past, clinicians often distinguished between typical and atypical clinical syndromes as a means of predicting the most likely microbial causes. A typical presentation consisted of the sudden onset of high fever, shaking, chills, and cough with purulent sputum. Such a presentation was considered more common with bacterial pathogens such as pneumococcus and H. influenzae. An atypical presentation was an illness characterized by the gradual onset of fever, headache, constitutional symptoms, diarrhea, and cough, often with minimal sputum production. Coughing was often a relatively minor symptom at the outset, and the illness was initially dominated by nonrespiratory symptoms. Such a presentation was thought to be more common with pathogens such as M. pneumoniae, C. pneumoniae, Legionella species, and viruses. More recent studies have shown that these distinctions are not ironclad and that considerable overlap exists in the clinical presentations of pneumonia with typical and atypical pathogens.17 The occurrence of concomitant diarrhea, previously considered indicative of legionellosis, is now known to be common in pneumococcal and mycoplasmal pneumonia. As noted previously, inhalation anthrax is a rare disease, but it warrants mention because of the small epidemic believed to have been an act of bioterrorism.15 This outbreak affected mainly postal workers who were exposed to mail containing anthrax spores. Most patients presented with a febrile flulike illness of several days’ duration accompanied by dry cough and shortness of breath. Some patients in whom the diagnosis was not quickly considered went on to develop septic shock, meningitis, and disseminated intravascular coagulation over several days, culminating in death. Because of a lack of prior host immunity or unique viral virulence factors, patients infected with pandemic influenza strains may have unusually severe presentations. During the 2009-2010 pandemic of H1N1 influenza, clinical presentations varied from mild upper respiratory syndromes to fulminant pneumonias with acute respiratory distress syndrome (ARDS) and shock.14 Severe acute respiratory syndrome manifests with high fever and myalgia for 3 to 7 days followed by nonproductive cough and progressive hypoxemia with progression to mechanical ventilation in 20%.16 A normal chest radiograph does not exclude the diagnosis of pneumonia. The chest radiograph may be normal in patients with early infection, dehydration, or P. jiroveci infection. The pattern of radiographic abnormality is not diagnostic of the causative agent, although specific radiographic findings should suggest specific microbial differential diagnoses (Table 22-4). TABLE 22-4 Radiographic Patterns Produced by Pathogens in Community-Acquired Pneumonia Many cases of community-acquired pneumonia can be managed successfully on an outpatient basis. The challenge for the clinician is to identify individuals at higher risk of morbidity and mortality for whom hospitalization is indicated. Over the past 20 years, numerous studies have analyzed risk factors for mortality in patients with community-acquired pneumonia.17–19 Risk factors predictive of a high risk of death are summarized in Box 22-1. Fine and associates19 performed a meta-analysis of 127 cohorts of patients with community-acquired pneumonia. The study examined risk factors for fatal outcome. The overall mortality for the 33,148 patients in these cohorts was 13.7%. Eleven prognostic variables were significantly associated with mortality, including male sex, absence of pleuritic chest pain, hypothermia, systolic hypotension, tachypnea, diabetes mellitus, cancer, neurologic disease, bacteremia, leukopenia, and multilobar infiltrates on chest radiograph. Mortality varied according to the infecting agent and was highest for P. aeruginosa (61.1%), Klebsiella species (35.7%), Escherichia coli (35.3%), and S. aureus (31.8%). Mortality rates for more common pathogens were lower but still substantial and included Legionella species (14.7%), S. pneumoniae (12.3%), C. pneumoniae (9.8%), and M. pneumoniae (1.4%).
Pulmonary Infections
 State the incidence of pneumonia in the United States and its economic impact.
State the incidence of pneumonia in the United States and its economic impact.
 Discuss the current classification scheme for pneumonia and be able to define hospital-acquired pneumonia, health care–associated pneumonia, and ventilator-associated pneumonia.
Discuss the current classification scheme for pneumonia and be able to define hospital-acquired pneumonia, health care–associated pneumonia, and ventilator-associated pneumonia.
 Recognize the pathophysiology and common causes of lower respiratory tract infections in specific clinical settings.
Recognize the pathophysiology and common causes of lower respiratory tract infections in specific clinical settings.
 List the common microbiologic organisms responsible for community-acquired and nosocomial pneumonias.
List the common microbiologic organisms responsible for community-acquired and nosocomial pneumonias.
 Describe the clinical findings seen in patients with pneumonia.
Describe the clinical findings seen in patients with pneumonia.
 State the radiographic findings seen in patients with pneumonia; state why some patients with pneumonia may have a normal chest radiograph.
State the radiographic findings seen in patients with pneumonia; state why some patients with pneumonia may have a normal chest radiograph.
 Describe the risk factors associated with increased morbidity and mortality in patients with pneumonia.
Describe the risk factors associated with increased morbidity and mortality in patients with pneumonia.
 State the criteria used to identify an adequate sputum sample for Gram stain and culture.
State the criteria used to identify an adequate sputum sample for Gram stain and culture.
 Describe the techniques used to identify the organism responsible for nosocomial pneumonia.
Describe the techniques used to identify the organism responsible for nosocomial pneumonia.
 List the latest recommendations regarding empiric and pathogen-specific antibiotic regimens used to treat various types of pneumonia.
List the latest recommendations regarding empiric and pathogen-specific antibiotic regimens used to treat various types of pneumonia.
 Discuss strategies that can be used to prevent pneumonia.
Discuss strategies that can be used to prevent pneumonia.
 Describe how the respiratory therapist aids in diagnosis and management of patients with suspected pneumonia.
Describe how the respiratory therapist aids in diagnosis and management of patients with suspected pneumonia.
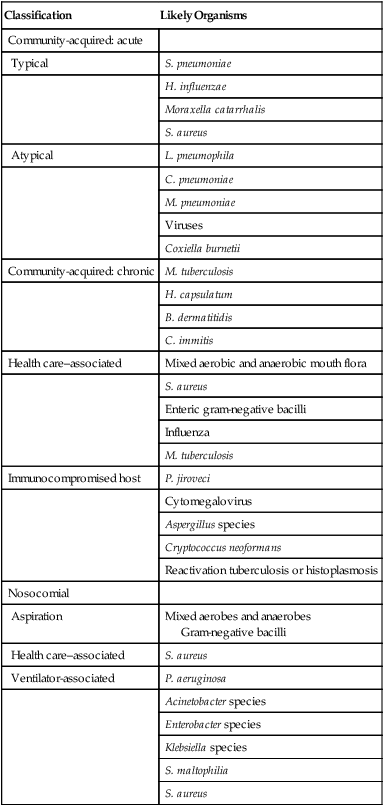
Classification
Classification
Likely Organisms
Community-acquired: acute
Typical
S. pneumoniae
H. influenzae
Moraxella catarrhalis
S. aureus
Atypical
L. pneumophila
C. pneumoniae
M. pneumoniae
Viruses
Coxiella burnetii
Community-acquired: chronic
M. tuberculosis
H. capsulatum
B. dermatitidis
C. immitis
Health care–associated
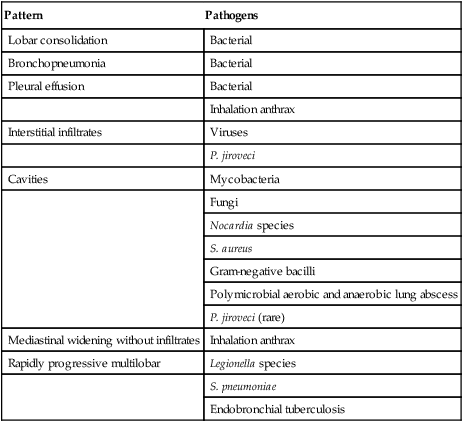
Mixed aerobic and anaerobic mouth flora
S. aureus
Enteric gram-negative bacilli
Influenza
M. tuberculosis
Immunocompromised host
P. jiroveci
Cytomegalovirus
Aspergillus species
Cryptococcus neoformans
Reactivation tuberculosis or histoplasmosis
Nosocomial
Aspiration
Mixed aerobes and anaerobes
Gram-negative bacilli
Health care–associated
S. aureus
Ventilator-associated
P. aeruginosa
Acinetobacter species
Enterobacter species
Klebsiella species
S. maltophilia
S. aureus
Pathogenesis
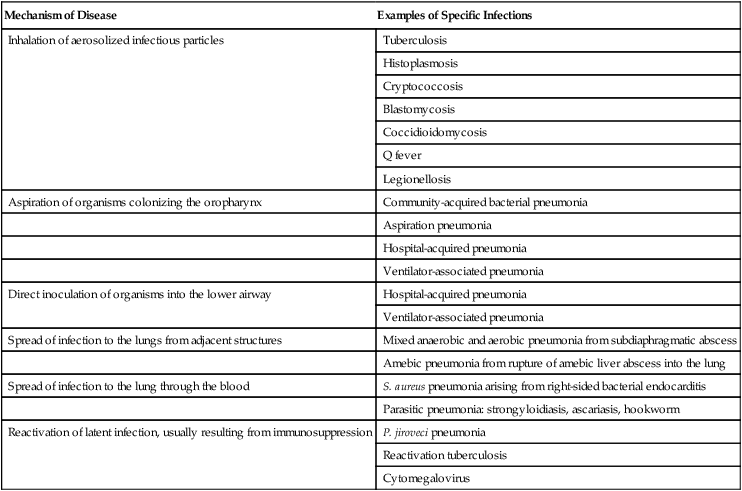
Mechanism of Disease
Examples of Specific Infections
Inhalation of aerosolized infectious particles
Tuberculosis
Histoplasmosis
Cryptococcosis
Blastomycosis
Coccidioidomycosis
Q fever
Legionellosis
Aspiration of organisms colonizing the oropharynx
Community-acquired bacterial pneumonia
Aspiration pneumonia
Hospital-acquired pneumonia
Ventilator-associated pneumonia
Direct inoculation of organisms into the lower airway
Hospital-acquired pneumonia
Ventilator-associated pneumonia
Spread of infection to the lungs from adjacent structures
Mixed anaerobic and aerobic pneumonia from subdiaphragmatic abscess
Amebic pneumonia from rupture of amebic liver abscess into the lung
Spread of infection to the lung through the blood
S. aureus pneumonia arising from right-sided bacterial endocarditis
Parasitic pneumonia: strongyloidiasis, ascariasis, hookworm
Reactivation of latent infection, usually resulting from immunosuppression
P. jiroveci pneumonia
Reactivation tuberculosis
Cytomegalovirus
Microbiology
Cause
Cases (%)
S. pneumoniae
20-75
Aspiration
6-10
C. pneumoniae
4-11
H. influenzae
3-10
Gram-negative bacilli
3-10
S. aureus
3-5
Legionella species
2-8
Viruses
2-16
Moraxella catarrhalis
1-3
M. pneumoniae
1-24
P. jiroveci
0-13
M. tuberculosis
0-5
No diagnosis
25-50
Clinical Manifestations
Chest Radiograph
Pattern
Pathogens
Lobar consolidation
Bacterial
Bronchopneumonia
Bacterial
Pleural effusion
Bacterial
Inhalation anthrax
Interstitial infiltrates
Viruses
P. jiroveci
Cavities
Mycobacteria
Fungi
Nocardia species
S. aureus
Gram-negative bacilli
Polymicrobial aerobic and anaerobic lung abscess
P. jiroveci (rare)
Mediastinal widening without infiltrates
Inhalation anthrax
Rapidly progressive multilobar
Legionella species
S. pneumoniae
Endobronchial tuberculosis
Risk Factors for Mortality and Assessing the Need for Hospitalization
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
