INDICATIONS
Patients with CTEPH usually present with dyspnea on exertion. A history of prior symptomatic pulmonary embolus is not readily documented in many of these patients. Without that history, the diagnosis can be particularly difficult to make and is often quite delayed. Not uncommonly, however, a patient will provide a history of having had a large pulmonary embolus. Typically the therapy for that event will have been entirely appropriate. Other patients may report that the diagnosis was not made until few months after the acute event or perhaps that therapy was suboptimal.
Patients then progress to hypoxemia and will ultimately manifest signs and symptoms of right heart failure. They may then present with ascites or lower extremity edema. This is obviously a sign of progression of the disease. It was formally believed that the reason for this progression was recurrent pulmonary emboli. Though this certainly may be true, a number of these patients will progress with vena caval filters in place and on appropriate anticoagulation. There appears to be a secondary fibroproliferative response within the pulmonary arteries, which develops producing progressive more distal obstructions.
Younger patients may be relatively asymptomatic at rest, but become profoundly short of breath with minimal exercise. This is explained by the presence of a fixed obstruction and the lack of a normal response to exercise. Patients with coronary disease may present with angina. Alternatively, excessive right heart strain can also produce angina even in the absence of significant coronary obstructions. Most patients will relate a history of lightheadedness or possible syncope if questioned carefully. This is more common following a cough or other maneuvers, which elicit a Valsalva and produce a transient increase in pulmonary vascular resistance.
Once the diagnosis has been established, proper selection of patients for surgical intervention can occur. Candidates for operation must have pulmonary vascular obstructive disease with significant hemodymanic and cardiopulmonary impairment. A pulmonary vascular resistance of at least 300 dynes/sec/cm-5 at rest or after exercise should be seen. The thrombi should also be surgically accessible. The development of a classification scheme of the anatomic distribution has been helpful in identifying appropriate patients. Type I disease is associated with obvious central thrombus. Type II disease demonstrates no major vessel thrombus but rather intimal thickening and webs at the main lobar and segmental level. Type III describes disease that is restricted to the segmental and subsegmental levels. Finally type IV disease affects only very peripheral resistance vessels and is nonoperative. Type I and II diseases are quite amenable to endarterectomy but type III disease should only be approached by the surgeon experienced in pulmonary endarterectomy.
Once mean pulmonary arterial (PA) pressures exceed 30 mm Hg, the 5-year survival is only 30%. Once that pressure exceeds 50 mm Hg, the 5-year survival is only 10%. The majority of the patients who we have cared for have had mean PA pressures averaging nearly 50 mm Hg. That would imply a 90% risk of death over the next 5 years. With our current perioperative survival rate of >95%, the risks and extensive nature of this surgical operation are justified and seen as quite acceptable by our patients.
 CONTRAINDICATIONS
CONTRAINDICATIONS
As stated above, patients with type IV disease should not undergo endarterectomy. Patients selected for endarterectomy should also have limited comorbidities. Significant parenchymal lung disease is a risk factor for prolonged postoperative mechanical ventilation, limited improvement in dyspnea, and increased perioperative mortality. Peripheral vascular disease is also a strong relative contraindication. Chronic renal insufficiency makes postoperative fluid management and establishment of a brisk and timely diuresis troublesome. Those with compromised renal function tend not to fare as well. When considering patients over the age of 80 one should be very selective.
Finally patients must understand and accept the risks of surgery. In addition to complications that are relatively common to open heart surgery, there are added risks associated with circulatory arrest and operating on patients in advanced stages of right heart failure. A generous amount of time is, therefore, spent with patients and their families counseling them on the specifics of the operation, the anticipated postoperative course and the potential complications. In general patients are very enthusiastic about surgical intervention after the natural history of their disease has been explained.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Without attention, the evaluation can quickly become redundant or misdirected. Therefore, it is important to organize existing information and order further studies with three specific goals in mind.
The goals are:
1. To determine whether the patient has pulmonary hypertension and assess its severity.
2. To determine the etiology of that pulmonary hypertension.
3. To determine if the patient’s disease is surgically accessible.
The diagnostic evaluation has often begun long before the patient presents to a surgeon and the first two goals are usually established. The surgeon role is to determine operability and surgical candidacy.
These are the diagnostic modalities that have become our standard routine:
Pulmonary Function Testing (PFT)
PFT is critical. More important than documenting any specific numbers or threshold values, one must be able to recognize the pattern of abnormalities. Specifically, the symptoms and gas exchange abnormalities are far greater than any spirometric defects. There is often a significant reduction in the diffusion capacity that goes with the patients’ experience of pronounced dyspnea. However, there are typically lesser restrictive or obstructive defects. Alternatively, patients with CTEPH may have other parenchymal lung disease that increases the risk of surgery or contraindicates it altogether.
ABGs
The PO2 on blood gas analysis is often low. If this is not the case at rest, there may be a precipitous decrease with exercise. This is due at least in part to patients’ inability to increase their cardiac output adequately with exercise because of fixed obstructions to pulmonary blood flow. As they consume oxygen with exercise, their mixed venous oxygen content drops. Since reoxygenation is limited by inadequate cardiac output traversing functioning lung, progressive hypoxemia ensues. Furthermore, up to 25% of these patients have a patent foramen ovale. With exercise, systemic vascular resistance drops and there is a rapid rise in PA and right heart pressures. The resultant right to left shunting, at the atrial level, will further exacerbate any hypoxemia.
Echocardiography
It provides an estimate of pulmonary pressures and also screens for any coexistent cardiac pathology. Right atrial and ventricular enlargement are typically obvious and both the atrial and ventricular septae may be displaced from right to left (Figs. 46.1 and 46.2). A patent foramen ovale in 25% of these patients however its detection may require an agitated saline contrast study. The tricuspid regurgitation is typically moderate to severe. Once the echocardiogram has indicated the presence of pulmonary hypertension the first goal of the diagnostic evaluation is essentially achieved. Next one must try and discern the etiology.
Ventilation–perfusion (VQ) Scan
VQ scanning typically demonstrates segmental or larger mismatched defects (Fig. 46.3). With primary pulmonary hypertension, the defects may be patchy or subsegmental or the scan may be entirely normal. This, therefore, allows a better distinction between small resistance vessel disease and chronic thromboembolic pulmonary hypertension. Unfortunately, VQ scanning tends to underestimate the degree of central obstruction. Recanalized vessels may offer hemodynamical resistance to flow but still allow isotope to reach the periphery. As a result scans may look deceptively normal. Furthermore, VQ scanning does not adequately demonstrate the magnitude, exact location or proximal extent of the thickening of the diseased arterial wall. It, therefore, is inappropriate to use VQ scanning alone to select patient for operation. VQ scanning is used however, as a screening tool to determine which patients should be appropriately referred for more invasive testing.
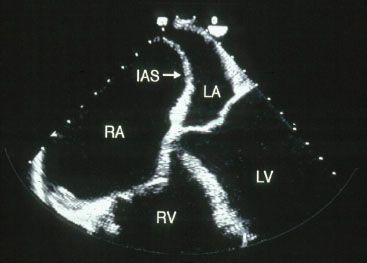
Figure 46.1 TEE showing enlarged right atrium.
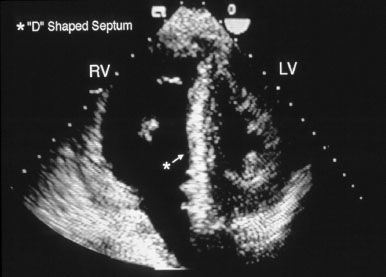
Figure 46.2 Echocardiogram showing bulging of the interventricular septum to the left (D-shaped left ventricle).
Right Heart Catheterization (RHC) and Pulmonary Angiography
Once pulmonary hypertension has been documented by echocardiography and an etiology has been suggested by VQ scanning, patients proceed to RHC and pulmonary angiography.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


