LEARNING OBJECTIVES
Learning Objectives
The student will be able to describe the clinical symptoms and signs of patients presenting with pulmonary embolism (PE).
The student will be able to define the pathophysiology of PE and the risk stratification of patients diagnosed with thromboembolism.
The student will be able to evaluate shock and right ventricular dysfunction as a discriminator of outcome during or after PE.
The student will be able to provide a stepwise approach to the treatment of patients diagnosed with acute PE.
The anatomical structure of the pulmonary vasculature predisposes to its ability to filter intravascular substances that develop within, or gain access to, the venous circulation. With respect to this filtering function, several clinical syndromes may occur depending upon the nature and quantity of the filtered intravascular materials as well as the physiological reserve of the individual. Although diverse substances including fat, air, amniotic fluid, tumor cells, and septic material can embolize to the lungs, acute pulmonary thromboembolism is the focus of this chapter because of its overwhelmingly predominant frequency and clinical importance.
ETIOLOGY AND EPIDEMIOLOGY OF PULMONARY EMBOLISM
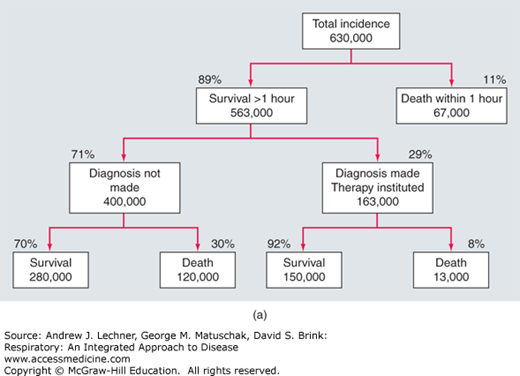
Pulmonary embolism and deep venous thrombosis (DVT) represent a continuum of the same underlying disorder of venous thromboembolism (VTE), the abnormal intravascular clotting within the venous system. Pulmonary thromboemboli originate from large thrombi in the deep veins of the lower extremities including the iliac, femoral, superficial femoral, and pelvic veins in 75%-90% of cases. The remaining pulmonary thromboemboli arise from venous thromboses of the upper extremities that are commonly associated with central venous or dialysis catheters, cardiac pacing wires, and other devices. Rates of VTE rise with increasing age, with an approximate doubling of risk over each 10-year interval. The estimated annual incidence of PE in the United States exceeds 600,000 cases, causing or contributing to death in up to 200,000 cases each year (Fig. 27.1). Overall, PE is the third most common cause of death in hospitalized patients. Approximately 10% of patients with acute PE die within the first hour. Subjects presenting with hemodynamic instability defined as right ventricular dysfunction and shock have a worse prognosis (Chaps. 28 and 29), and manifest an in-hospital mortality rate as high as 30%, primarily as a result of recurrent embolism. However, accurate diagnosis and effective treatment significantly reduce the mortality rate to 2%-8%.
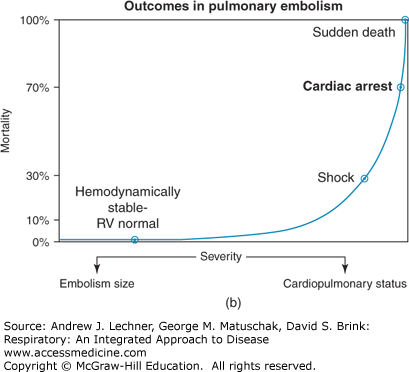
FIGURE 27.1
(a) Of the ~200,000 deaths/yr from PE in the United States, 13,000 occur in patients who received treatment, while 94% of patients who die of PE do not receive treatment because the diagnosis is not made. (b) The relationship of severity and mortality in patients with multiple or severe PE. Adapted from Wood et al. Major pulmonary embolism: review of a pathophysiological approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877-905.
RISK FACTORS FOR PULMONARY EMBOLI
As described by the German pathologist Rudolf Virchow in 1856, the major events that predispose to the development of VTE are summarized by Virchow’s triad: venous stasis as occurs after prolonged immobilization; endothelial injury of the vessel wall; and hypercoagulability with activation of the clotting system. Because most clinically significant PE emanate from the deep veins of the lower extremities, the major risk factors for VTE are those processes that predispose a patient to the development of DVT. Accordingly, the likelihood of developing VTE is collectively increased by a post-operative state with prolonged bed rest or immobilization, trauma to the blood vessel wall, and acquired or hereditary risk factors for hypercoagulability.
Orthopedic surgery of the lower extremity has long been recognized as one of the strong risk factors for VTE. Thus, DVT may develop in >50% of patients who do not receive prophylactic anticoagulant therapy while undergoing elective hip or knee replacement surgery. In such cases, >90% of proximal thrombi in hip replacement patients occur ipsilaterally to the operated site. Other acquired risk factors include major trauma, abdominal surgery requiring >30 minutes of anesthesia, spinal cord injury with associated paralysis of the lower extremities, the antiphospholipid antibody syndrome, advanced malignancy, and the post-partum state (Table 27.1).
| Strong Risk Factors | Moderate Risk Factors |
|---|---|
| Fracture of hip, pelvis, or leg | Prior VTE |
| Knee or hip replacement surgery | Postpartum period |
| Major traumatic injury | Malignancy |
| Spinal cord injury | Estrogen therapy, oral contraceptives |
| Antiphospholipid antibody syndrome | Congestive heart failure |
| Weak Risk Factors | Stroke |
| Advanced age >60 years | Respiratory failure |
| Obesity | Indwelling central venous catheter |
| Antepartum period | Arthroscopic knee surgery |
| Varicose veins |
CLINICAL CORRELATION 27.1
There is nearly an eightfold increased risk for acute VTE in patients with a history of VTE who undergo major surgery, who experience periods of immobility, or who are hospitalized for medical illnesses, compared with patients without such a history. These patients, and others with risk factors, must be aggressively targeted for prophylactic therapy.
Hereditary abnormalities of coagulation termed thrombophilias further constitute recognized risk factors for VTE and PE (Table 27.2). These hereditary thrombophilias by themselves not only predispose to VTE, but interact synergistically with acquired surgical, medical, obstetric, and other risk factors (Table 27.1) to amplify the likelihood of developing VTE.
Such inherited thrombophilias should be suspected when a patient has recurrent or life-threatening VTE and PE, has a family history of VTE, is younger than 45 years of age, and has few or no medical or surgical risk factors. Female patients with inherited thrombophilias may furthermore give a history of multiple spontaneous abortions, stillbirths, or both. The most clinically important hereditary thrombophilia associated with VTE in up to 40% of cases is the Factor V Leiden mutation. In such patients, the mutated activated Factor V (Factor Va) is resistant to the anticoagulant effects of protein C, or in other words is responsible for protein C resistance. Heterozygosity for the Factor V mutation increases the risk of VTE by 5- to 10-fold, whereas homozygosity increases risk by approximately 80-fold.
A 68-year-old obese male presented to the emergency department with lightheadedness progressing to near-syncope and progressive shortness of breath of three days’ duration. His symptoms started a week ago when he first experienced pleuritic chest pain that radiated to his right side. He had undergone sinus surgery 3 weeks prior to presentation. His past medical history was significant for hypertension, obstructive sleep apnea, and diastolic heart failure; a DVT following knee surgery had occurred 15 years previously. He quit smoking 20 years ago and did not report alcohol use. Physical exam revealed tachypnea, sinus tachycardia at a HR = 118 beats/min, BP = 98/62 mm Hg, and Sao2 = 90% while on 6 L/min of supplemental inspired O2. Auscultation of the chest showed scattered wheezes and left basilar crackles (Chap. 14). No calf tenderness was present. Given the high clinical suspicion for acute PE, a CT angiogram of the chest was performed that revealed multiple filling defects (Fig. 27.2) (see Chap. 15). The patient was thereafter anticoagulated with low-molecular weight heparin (LMWH).
CLINICAL PRESENTATION OF PULMONARY EMBOLISM
Acute PE may variably present with symptoms ranging from mild acute, unexplained dyspnea to sustained shock. On occasion, the patient may be asymptomatic or have relatively nonspecific symptoms and be diagnosed only by imaging procedures performed for other purposes. In the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study of patients with documented PE, dyspnea was present in 73% of cases and pleuritic chest pain in 66%, whereas hemoptysis occurred in only 13% of patients. Thus, the classic triad of the sudden onset of dyspnea, pleuritic chest pain, and hemoptysis actually occurs in a minority of cases. Dyspnea is thought to result from reflex bronchoconstriction secondary to release of endogenous mediators as well as increased pulmonary artery pressure, reduced pulmonary compliance because of surfactant depletion, and stimulation of pulmonary C-fibers (Chaps. 5, 6, 10, and 11). In patients with large thromboemboli or preexisting pulmonary comorbidities, right ventricular strain may furthermore be contributory to the sensation of dyspnea. Hemoptysis is more typically noted with pulmonary infarction but may also result from transmission of systemic arterial pressure to the pulmonary microvasculature via bronchopulmonary anastomoses, with subsequent capillary disruption. Alternatively, hemoptysis may reflect hemorrhagic pulmonary edema from surfactant depletion or neutrophil-associated capillary injury (Chaps. 10 and 28).
Regarding signs in acute PE, tachypnea that is characterized by a respiratory f >20 breaths/min is observed in 70% of patients, with crackles noted in 51% (Chap. 14). Cardiovascular manifestations are less common, with tachycardia characterized by a HR >100 beats/min observed in 30%, an S4 gallop rhythm in 24%, and an accentuated pulmonic component of the second heart sound in 23%. At least 70% of patients with PE do not have leg symptoms or signs at the time of diagnosis, as noted in the clinical vignette above. On the other hand, asymptomatic PE occurs in up to one-half of patients with leg DVT. Overall, the important point to be noted is that these discussed symptoms and signs are neither sensitive nor specific, and thus can be found both in patients with PE and without PE in conjunction with other cardiopulmonary conditions. Therefore, an imaging evaluation is always necessary to diagnose or exclude PE.
DIFFERENTIAL DIAGNOSIS
Considering that symptoms and signs in PE are frequently nonspecific, other diagnoses must be evaluated (Table 27.3). Chest radiography is useful in diagnosing pneumonia, pleural effusions, pneumothorax, or pulmonary edema of cardiogenic or noncardiogenic origin, all of which can present with dyspnea or chest pain. Myocardial ischemia may present with acute chest pain or pressure associated with dyspnea. Also, based on prior clinical history, exacerbations of asthma or COPD, gastroesophageal reflux disease, and esophageal dysmotility are differential diagnostic possibilities.
NATURAL HISTORY OF VENOUS THROMBOEMBOLI
As noted earlier, the lungs are the most common target organ for intravascular emboli because the entire cardiac output passes through the pulmonary vasculature each minute. In massive pulmonary thromboembolism that causes sudden death
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree