Hemodynamic Monitoring
Patients who need pulmonary angiography are often acutely ill and may require continuous blood pressure measurements and electrocardiographic monitoring. Sinus bradycardia or heart block may occur as vascular access is gained. Complete heart block during right heart catheterization can also occur owing to impact of the right bundle branch in patients with underlying left bundle branch block, rarely necessitating temporary pacing. Transient supraventricular and ventricular arrhythmias are also common during catheter advancement through the right heart chambers, and sustained tachyarrhythmias with hemodynamic impairment may necessitate electrical cardioversion.
An important part of the procedure is formal hemodynamic measurements (both pressures and oxygen saturation) during catheter advancement. The coronary sinus is occasionally entered while trying to access the right ventricular outflow tract (particularly from subclavian, jugular,
or brachial access route). To minimize the risk of perforation, catheter advancement should be halted if a right atrial pressure waveform continues to be present as the catheter is advanced across the spine into what should fluoroscopically be the right ventricle. Catheter position in the coronary sinus can be confirmed or excluded by a hand injection of a contrast medium under fluoroscopy. Damping of the pressure in the main pulmonary artery may indicate the presence of massive pulmonary embolism (PE), with the catheter holes embedded in the embolus. In that situation, a hand injection of contrast can confirm the diagnosis.
The formal hemodynamics prior to angiography (
Table 18.1) may also suggest the presence of congestive heart failure, valvular disease, intracardiac shunts, pulmonary hypertension, or pericardial disease. Severe hemodynamic embarrassment may also require modification of the angiographic procedure, including catheter placement, injection rates, and image recording modes. In particular, complications of pulmonary angiography are more common in patients with pulmonary hypertension (particularly in the presence of right ventricular dysfunction), mandating special precautions such as supplemental oxygen, reduced amounts of contrast agent, or superselective rather than mainstream pulmonary artery injections.
6
Percutaneous Venous Catheterization
Pulmonary angiography is performed using the technique described by Seldinger in 1953.
7 The veins used for catheterization of the pulmonary artery are the femoral, jugular and upper extremity vein. Of these, the right femoral vein is preferable because it provides a relatively straight course to the inferior vena cava and right heart. In patients with suspected proximal deep vein thrombosis (DVT), ultrasound examination may be considered prior to vascular entry. The procedure is performed with mild conscious sedation. It is important
that the patient be alert during the procedure so that he can cooperate with breath holding during imaging. In case heparin has been administered for suspected pulmonary embolism, it should be continued during the examination.
The technique for arterial and venous vascular access has been described in detail in
Chapter 6, and the reader is referred to that discussion. To minimize the risk of dislodging thrombi during catheter advancement,
8 manual injection of 10 to 15 mL of contrast into the femoral vein may help to exclude massive iliac vein or cava thrombosis prior to advancing the catheter to the right heart.
Occasionally, because of femoral or iliac vein thrombosis, inferior vena cava occlusion, or groin infection, the femoral vein cannot be used. The vein of choice then becomes the jugular or an upper extremity vein. The right heart may be approached easily with a balloon-directed catheter when gaining vascular access via the internal jugular vein.
Of the upper extremity veins, the basilic vein in the antecubital fossa is preferable, while the cephalic vein is not suitable since it enters the axillary vein at an abrupt angle. If the basilic vein cannot be accessed, the brachial vein can also provide access.
Pulmonary Artery Catheterization
Most catheters used for diagnostic pulmonary angiography are between 5F and 7F to provide a lumen that will accommodate contrast injection rates of 20 to 25 mL/second.
9 A 4F nylon pulmonary catheter allows flow rates of 20 mL/ second at 1,050 psi
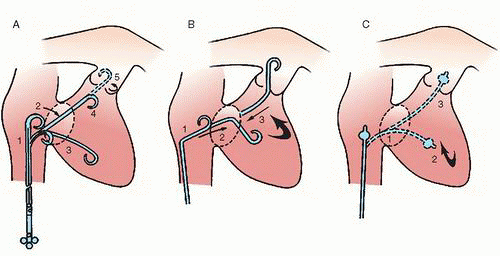
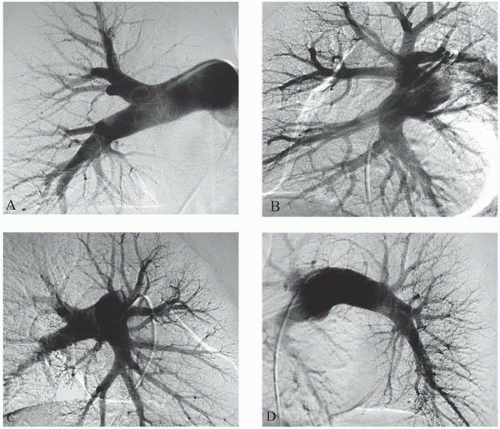
10 and may reduce access site complications. The three common approaches for pulmonary artery catheterization are shown in
Figure 18.3. The presence of a properly placed IVC filter does not necessarily preclude a transfemoral approach. Safe transfilter angiography has been reported by passing straight or J-tipped guide wires followed by catheters through stainless steel Greenfield, Vena Tech, and Bird’s Nest filters.
11 After the guide wire is passed through the IVC filter, a long sheath is placed across the filter with its leading tip beyond the filter to prevent filter dislodgment.
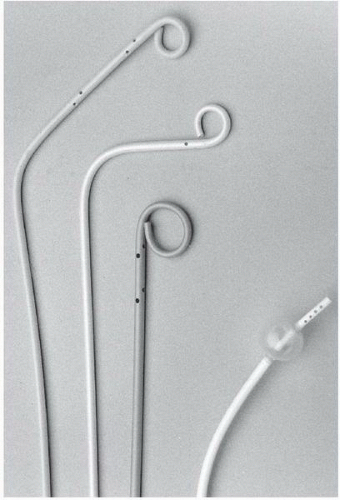
Catheters used for pulmonary angiography are of two basic designs: the pigtail type and balloon-tipped type. The pigtail type catheters have multiple side holes whereas the curled catheter tip allows safe passage through the right heart. While being removed from the pulmonary arteries, all pigtail catheters must be straightened with a floppytip guide wire or a J-tipped guide wire under fluoroscopic observation, since the catheter tip may otherwise engage a papillary muscle, chordae tendineae, or tricuspid valve leaflet during withdrawal. The balloon-tipped catheters are assisted by blood flow through the right heart chambers and into the pulmonary arteries. Side holes in the catheter shaft allow power injection into the main branches, whereas the catheter end-hole makes balloon occlusion angiography possible with the same catheter (
Figure 18.4).
Balloon catheters are first deflated and can then be removed without fluoroscopy.
The most common pigtail catheter is the Grollman pulmonary artery catheter (Cook Inc. Bloomington, IN). This 6.7F polyethylene catheter has a 90° reversed secondary curve 3 cm proximal to the pigtail
12 (
Figure 18.4). If the catheter tip becomes lodged in the right ventricular outflow tract, use of a soft-tipped J guide wire may facilitate catheter entry into the main pulmonary artery. In challenging cases, the pulmonary artery can be catheterized using a conventional large-lumen balloon flotation catheter with placement of an exchange-length J-tipped guide wire in the pulmonary artery, and subsequent advancement of the angiographic pigtail over the wire.
In patients with right atrial enlargement, the right ventricle may be difficult to probe with the standard Grollman catheter because the distal end of the catheter may be too short to allow direct passage. In such cases, the 90° angle of the distal tip may be enlarged by introducing a manually bent proximal end of a guide wire.
13 The Van Aman (7 Fr APC, Cook Inc. Bloomington, IN) catheter is a 7F polyurethane modified Grollman catheter with a 90° reversed secondary curve 6 cm (rather than 3 cm) proximal to the pigtail and has been successfully used for pulmonary artery catheterization in patients with right heart enlargement.
14The 7F Berman balloon catheter (Critikon Inc. Tampa, FL), which has no end hole, cannot be used with a guide wire, and requires introduction through a venous sheath. From the jugular or brachial approach, the catheter follows a continuous curve through the outflow tract and into the right pulmonary artery. The right pulmonary artery may be catheterized from below by using a reverse curve in which the Berman catheter is curved against the lateral right atrial wall before crossing the tricuspid valve, so that it enters the right ventricle pointing up as though it were coming from above. This approach is particularly helpful in the presence of tricuspid regurgitation, since the right atrial catheter loop provides more backup when advancing the catheter than seen
with direct transit of the tricuspid valve from below. Catheterization of the left pulmonary artery is often more difficult, and may require the use of deflection guide wires into the angiographic catheter if standard attempts at catheter manipulation are unsuccessful.
Preferred catheters for the brachial approach include a 5F nonreversed Grollman catheter and a 5F multiple-bend pigtail catheter
15,
16 (Cordis Corp., Miami, FL). Direct catheter entry into the right ventricle may be difficult using the brachial approach. Looping the catheter around the right atrial free wall, counterclockwise rotation, and gentle retraction are necessary to probe the right ventricle.
The two catheters used for pulmonary angiography at the author’s institution are 7F curved pigtail catheter (7F APC, flow rate 32 cc/second at 1,200 psi) and 7F Mont-1 Torcon NB Advantage Catheter (flow rate 29 cc/second at 1,200 psi; Cook Medical Inc., Bloomington, IN). The 7F catheter can be introduced from a femoral or jugular vein without placing a 7F sheath in the vein. The tip of the catheter is turned toward the right ventricle just above the diaphragm. The catheter is advanced through the tricuspid valve until it enters the right ventricle, where the catheter is turned clockwise while advancing it toward the pulmonary outflow tract. Although pulmonary artery catheterization with the curved pigtail catheter is generally easy, it may become difficult in patients with large right atrium and ventricle; in these patients, the curved catheter tip may not negotiate the tricuspid valve. In such patients, the tip-deflecting wire technique is used to advance the catheter into the right ventricle. The deflecting wire is positioned in the catheter just proximal to the pigtail. The wire is deflected, directing the catheter toward the tricuspid valve, and then the manipulator instrument is held stable. The catheter is advanced off the manipulator wire into the right ventricle. When the catheter tip is in the right ventricle, the manipulator wire is withdrawn, and then the catheter is advanced into the right ventricular outflow tract and pulmonary artery while rotating it clockwise. Alternatively, a guide wire can be advanced through the catheter into the right ventricle and pulmonary artery. If the catheter tip is being advanced toward the right ventricular apex, causing ventricular arrhythmias, it should be retracted immediately toward the tricuspid valve, and then a J-tipped guide wire should be advanced into the pulmonary artery. The catheter is then advanced into the pulmonary artery over the guide wire. When the catheter tip is advanced from the cephalic portion of the right atrium, occasionally it will pass through a patent foramen ovale or an atrial septal defect into the left atrium and even into the pulmonary vein. In such a situation, the injection of contrast medium into the pulmonary vein will fill the left atrium without filling the pulmonary vasculature. When this occurs, the catheter tip is withdrawn to the right atrium and re-advanced from the caudal portion of the right atrium into the right ventricle and then into the pulmonary artery. Occasionally, the catheter tip will enter the coronary sinus without entering the right ventricle. When this occurs, the tip of the catheter will not advance. The catheter tip should then be withdrawn into the right atrium, and re-advanced into the right ventricle.
Once the catheter is positioned in the left pulmonary artery, it can be connected to a pressure transducer and the pulmonary artery pressure can be measured. After the pressure is obtained, selective pulmonary angiography is performed in two oblique projections. The catheter is then turned toward the right pulmonary artery while retracting it to the main pulmonary artery. If this maneuver fails to reposition the catheter in the right pulmonary artery, a standard guide wire or a tip-deflecting wire technique can be used to turn the catheter tip from the left pulmonary artery to the right pulmonary artery.
Contrast Agents and Injection Rates
Low-osmolar contrast agents with an iodine concentration of at least 300 mg/mL are recommended for pulmonary angiography. The achieved reduction in side effects such as cough reflex, flushing, hypotension, and nausea with these nonionic agents promotes motion-free image acquisition.
17In vitro activation of platelets has been reported with the low-osmolar agents iohexol (Omnipaque, GE Healthcare Inc.) and iopamidol (Isovue, Bracco Diagnostics).
18 One study found increased plasma levels of plasminogen activator inhibitor-1 in patients following pulmonary angiography with iohexol and increased thrombin-antithrombin III complexes with iohexol and ioxaglate (Hexabrix, Guerbet LLL).
19 Newer isosmolar nonionic agents have not been tested in patients undergoing pulmonary angiography, but the isosmolar nonionic dimer iodixanol (Visipaque, GE Healthcare) appears to reduce major adverse cardiovascular events, as compared with low-osmolar ionic contrast agents, in patients with acute coronary syndromes who undergo percutaneous coronary intervention,
20 and also to reduce contrast-induced nephropathy, as compared with iohexol.
21The contrast injection rate is determined by the rate of blood flow in the selected vessel, pulmonary artery pressure, imaging modes, and the catheter used for angiography. Contrast medium should be injected at a rate that approximates as closely as possible the rate of blood flow in the artery being opacified. Injecting too slowly results in poor opacification of the pulmonary arterial trees. Too rapid an injection, on the other hand, results in reflux of the contrast medium into the contralateral pulmonary artery. The left and right pulmonary arteries have a blood flow of 25 cc per second in most patients. The injection rates are adjusted according to the flow rate estimated at test injections and the disease being investigated. The usual injection rates in patients with normal pulmonary artery pressure are 25 cc per second for a total volume of 50 cc. In general, the rate of injection for superselective pulmonary angiograms should be slightly more than the expected blood flow of the artery being injected to, to ensure complete filling of the vascular bed. Depending on the size of the pulmonary artery being injected to, the injection rate for superselective angiogram is 5 to 10 cc per second for a total volume of 15 to 20 cc (
Table 18.2). In the presence of pulmonary hypertension, the amount of contrast medium should be reduced to minimize the adverse hemodynamic impact of a full contrast injection under such circumstances.
22 The rate of injection in this condition should be reduced to 15 to 20 cc per second for a total volume of 30 to 40 cc. Even though the rate of injection is reduced, the total volume of contrast should be at least 30 cc to ensure complete filling of the central pulmonary arteries for the evaluation of pulmonary thromboembolic disease. With the use of low-osmolar contrast agents, tailored pulmonary angiography with lower flow rates and more distal injections has proved to be safe even in the presence of severe pulmonary hypertension. Contrast injection should be performed using an automated injector system at a pressure of at least 600 psi (42 kg/cm
2). For balloon occlusion angiography of segmental vessels, a hand injection of 5 to 10 mL is used.
Imaging Modes
Digital techniques have virtually replaced conventional cut films. Hagspiel et al.
23 found digital subtraction angiography
(DSA) with selective pulmonary arterial injections equivalent to conventional cut-film angiography in diagnostic performance and image quality. In 80 patients, DSA allowed more accurate detection of pulmonary emboli with better interobserver agreement than allowed by conventional cut film.
24 In 54 patients with suspected PE but a negative digital angiogram, none suffered a thromboembolic event after a mean of 12 months.
25The major advantage of DSA over cut film is that highresolution images can be obtained with lesser amount of contrast agent. This is particularly important for evaluation of patients with pulmonary hypertension and renal insufficiency. Other advantages include rapid image acquisition and flexible display format. Images can be viewed individually or in cine format on the monitor, in either the subtracted or the unsubtracted mode. Masks can be selected image by image and their pixels shifted to best match the anatomy. In addition, DSA may even allow satisfactory opacification of pulmonary arteries when contrast is injected into the superior vena cava or right atrium. The major disadvantage of DSA is that it requires motionless image acquisition. This may be especially difficult in evaluation of patients with severe cardiopulmonary symptoms, who may not be able to hold their breath during image acquisition. Mask shifting helps minimize cardiac motion artifacts but is less helpful in reducing respiratory motion artifacts. However, although serial cut film still offers higher spatial resolution than that achieved by cineradiography or DSA, there is no evidence that DSA is inferior to serial cut film in the detection of subsegmental PE.
Filming rates are based on the normal transit rate of contrast through the lung. Injected contrast reaches the capillaries in 2 to 3 seconds while the left atrium fills in 4 to 6 seconds.
26 With cut film, a total of 12 images are usually obtained: 3 images per second in 3 seconds, and 1 image per second for an additional 6 seconds. With digital systems, a full second of mask images are obtained before injection (about one cardiac cycle), with continued acquisition at the rate of 6 images per second. Higher rates may be used in uncooperative patients, in large individuals, or in situations where high flow is expected (for example in PAVMs). Slower acquisition rates are recommended for patients with low cardiac output.
A minimum of two radiographic series are required for each lung to exclude pulmonary embolism. The two standard views are the frontal and the 45° ipsilateral posterior oblique. These views have been validated for pulmonary embolism in a large clinical trial.
27 If it is available,
biplane filming is preferred over monoplane filming to reduce the total amount of contrast. Although the lateral is the true orthogonal view to the frontal projection, it is not desirable for most cases of pulmonary angiography, since even selective right or left injections frequently cause reflux into the opposite lung, which may confuse interpretation. If a sufficient amount of contrast (40 to 50 mL) is injected and prolonged filming is carried out, the lateral and oblique views may also be used to evaluate left ventricular size and function as well as the anatomy of the ascending aorta or proximal coronary arteries.
At the author’s institution, pulmonary angiograms are obtained with contrast injection in the right or left pulmonary artery. Left pulmonary angiography is performed in 50° right anterior oblique (RAO) and 40° LAO views. Right pulmonary angiography is performed in 30° RAO and 40° LAO views (
Figure 18.5). The DSA matrix should be the highest, at 1,024 × 1,024, whenever possible. For the field size, maximum magnification that allows visualization of the entire lung on both views should be used for obtaining the best images.