■ Acute upper extremity ischemia is less common than in the lower extremity due to the rich preexisting collateral circulation in both the upper arm and forearm. The majority (~50%) of acute ischemic complications in the upper extremity occur in elderly females as a result of embolic phenomenon rather than primary vessel thrombosis (which accounts for ~25% of acute ischemic events).3 The differential diagnosis for embolic sources includes intracardiac sources, proximal arterial atherosclerotic plaque, proximal arterial aneurysm thrombus, endocarditis, or paradoxical embolus from venous circulation.
■ Chronic arterial occlusive disease is rarely symptomatic. Associated comorbid conditions include diabetes, chronic atherosclerotic occlusive disease, subclavian or arteriovenous steal syndromes, or failure of prior arterial repair or grafting.4,5
■ Venous occlusive disorders in the upper extremity are common and are usually associated with iatrogenic injury, indwelling catheters, or thoracic outlet pathology. For further information regarding venous thoracic outlet disorders. Distal to the thoracic outlet, venous occlusive disorders are for the most part managed expectantly with anticoagulation therapy. Open surgical and endovascular therapies are rarely used and, due to high recurrence and failure rates, are not enthusiastically recommended. For further recommendations regarding upper extremity venous disease management, please refer to additional references.6,7
PATIENT HISTORY AND PHYSICAL FINDINGS
■ Initial evaluation should include an assessment of associated comorbidities, including cardiac pathologies (myocardial infarction, arrhythmia, heart failure, or prior coronary artery revascularization), hypertension, hyperlipidemia, diabetes, hypercoagulability, smoking, prior upper or lower extremity arterial intervention, or index extremity trauma.
■ Symptoms and signs of acute arterial ischemia include pain, paresthesia, pulselessness, paralysis, and/or poikilothermia. A thorough vascular, sensory, and motor examination will help assess the severity of arterial insufficiency. Although dated, the Rutherford classification system remains useful for prognostic determinations.8 For classes 5 and 6 ischemia, urgent or emergent intervention may be necessary to preserve limb function and viability. Prompt removal of embolic debris in limbs with sufficient residual viability produces excellent long-term results.3
■ Chronic limb ischemia may also present with symptoms of rest pain, pain with extremity use, paresthesia, pulselessness, poikilothermia, and/or ulcerations/wounds/gangrene of fingers or fingertips. Subjects should be asked to describe what, if any, activities exacerbate these potential symptoms (i.e., lifting or carrying material with affected arm/hand, arm raising, or repetitive arm/hand movement). Patients with vocational or recreational activities that require regular or frequent use of their upper extremities should describe convincing symptoms they experience in relation to these activities. More commonly, chronic upper extremity arterial ischemia is asymptomatic, particularly in older and less physically active individuals. In general, revascularization is not necessarily indicated in these circumstances. Discrepancy in upper extremity pulses, or brachial blood pressure differential of more than 15 mmHg, is a hallmark of chronic upper extremity arterial insufficiency with or without accompanying symptoms.
■ Traumatic or iatrogenic injury accounts for 25% of patients presenting with acute upper extremity arterial insufficiency. Consideration of the mechanism of injury (blunt, penetrating, hyperextension, or avulsion) will help delineate the likely nature of the resulting arterial disruption (transection, dissection, or thrombosis, with or without ongoing extravasation). Following completion of the trauma primary survey, determination of extremity arterial continuity should be performed following reduction of obvious ipsilateral upper extremity fractures and dislocations. In complex injuries, including avulsions and crush injuries, baseline sensory and motor status should be documented early to formulate the most appropriate course of therapy. When severe arterial injury is associated with transection or avulsion of the brachial plexus and compound long bone fractures, meaningful functional recovery, despite ultimately successful revascularization, may not be possible.9
■ More commonly, upper extremity arterial injuries can be more subtle and are frequently missed on primary or secondary surveys. The extensive collateral network present around the elbow often masks the presence of brachial artery thrombosis following posterior elbow dislocation, or dissection and thrombosis following brachial artery catheterization. When physical signs suggest asymmetric or reduced upper extremity arterial perfusion, objective imaging should be obtained promptly to direct therapy and maximize long-term function. Depending on hand perfusion and viability, occasionally, immediate revascularization can be at least temporarily deferred to allow for more urgent resuscitation and stabilization procedures to proceed. Similarly, non–flow-limiting dissections may be monitored without immediate intervention, particularly when the patient’s overall condition merits observational management.
IMAGING AND OTHER DIAGNOSTIC STUDIES
■ Depending on clinical circumstances, revascularization may be undertaken on the basis of clinically apparent injuries and limb ischemia. More commonly, physiologic assessment is indicated and necessary to determine the most efficacious method of revascularization. Arteriography, typically performed during or immediately prior to revascularization, remains an essential tool to guide intervention and confirm procedural success. As a general observation, physiologic testing helps determine when intervention is necessary, whereas arteriography provides the necessary anatomic information to ensure procedural and functional success.
■ Computed tomography (CT) arteriography can potentially add useful information to surgical planning for upper extremity revascularization. However, unlike in the abdomen and lower extremities, significant insight into disease localization and severity in the upper extremities can be gleaned from physical examination and nonionizing imaging modalities such as ultrasound. The potential additional diagnostic benefit associated with CT angiography needs to be balanced with the not insignificant radiation dosage delivered with this imaging modality, particularly in regard to the longer life expectancy of younger patients. When vascular disease is known to be limited to the extrathoracic upper extremity arterial system, CT angiography provides little additional, useful information over diagnostic arteriography alone, especially when the latter can be paired with a therapeutic intervention.
■ Noninvasive vascular testing for evaluation of the upper extremities includes segmental systolic pressure measurements using a Doppler flow detector, digital plethysmography, and arterial duplex scanning.
■ Serologic tests are the basis for the workup of patients with suspected vasculitic pathologies (e.g., Takayasu’s arteritis, giant cell arteritis, Buerger’s disease, and/or scleroderma). Customary tests include baseline complete blood count, platelets, fibrinogen, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR).10,11 In certain circumstances, color duplex evaluation may also provide additional clues to aid in the diagnosis.12 Additional serial serologies and rheumatologic consultation may be necessary depending on the severity and progression of the suspected vasculitic disease process.
■ Young patients with acute primary arterial thrombosis, patients with recurrent arterial thrombosis, or patients with a prior history of unprovoked deep venous thrombosis preceding an arterial event should be considered for a hypercoagulability workup and hematology consultation. Initial testing may include protein C function, free protein S, antithrombin III activity, anticardiolipin antibodies, factor V Leiden mutation, prothrombin mutation, and homocysteine level.13
SURGICAL MANAGEMENT
Preoperative Planning
■ Prior to attempted upper extremity arterial repair or revascularization, a clear understanding of the extent and location of arterial pathology is essential. This knowledge guides the location of arterial exposure or optimal method of arterial access, the identification of optimal inflow sources and outflow targets, and the most effective and efficient methods of reconstruction. When uncertainty persists, intraoperative arteriography provides essential and timely guidance.
■ As in all methods of peripheral arterial reconstruction, heparin is typically administered when an interventional sheath is first placed, or arterial control is anticipated. Typically, 100 units/kg of intravenous heparin is administered, with additional anticoagulation guided by the activated clotting time monitored during the course of the procedure.
■ As discussed in Chapter 2, the preferred extrathoracic method of innominate or proximal left subclavian artery surgical reconstruction is carotid subclavian bypass. Frequently, however, endovascular options are available and preferable in patients who cannot tolerate the risks or morbidity associated with open reconstruction. For example, in the setting of uncontrolled, life-threatening hemorrhage from penetrating or crush injuries or limb avulsion, bleeding may be controlled by insertion of covered, self-expanding stents across the area of injury in the subclavian or axillary arteries. However, with few other exceptions (such as lesions associated with giant cell or Takayasu’s arteritis), angioplasty and stenting of arterial lesions at or distal to the clavicle is poorly tolerated and ill advised. Stents placed in this area are at high risk for fragmentation and subsequent arterial thrombosis, pseudoaneurysm formation, or stent migration.
■ Surgical management of arterial pathology distal to the subclavian artery (surgical management of arterial disease of the arch vessels and subclavian artery are discussed in Chapters 1, 2, and 9) and proximal to the wrist (surgical management of arterial disease distal to the wrist is discussed in Chapter 11) will depend on acuity, cause/type of pathology (penetrating trauma, blunt trauma, occlusion, stenosis, or aneurysm), severity of patient symptoms, patient comorbidities, and required durability of the planned repair.
■ Axillary artery exposure is guided by the nature of the planned reconstruction. The proximal artery is most easily exposed via a transverse infraclavicular incision. Exposure of the second and third portions requires deltopectoral or axillary approaches, respectively.1
■ Acute symptomatic embolic occlusion of the axillary or brachial arteries are best managed by open, preferably image-guided, balloon catheter or direct thromboembolectomy.1,2 Essential elements required for thromboembolectomy include (1) determination of the optimal treatment environment (operating room [OR] with portable vs. fixed imaging), (2) arterial access (level of incision), (3) acquisition of catheters and guidewires required to transverse the embolus and accumulated luminal thrombus, (4) availability of balloon and over-the-wire embolectomy catheters, (5) need for adjuncts such as aspiration catheters (Export™ catheter, Medtronic, Minneapolis, MN) and thrombolytic agents (tissue plasminogen activator [tPA]), (6) options for managing postischemic hyperemia and elevated compartment pressures, and (7) consideration of treatment alternatives should preexisting atherosclerotic occlusive disease preclude or complicate catheter-directed thromboembolectomy.
■ For symptomatic axillary or brachial artery thrombosis not amenable to direct or catheter-based, image-guided thromboembolectomy, open surgical bypass or interposition grafting is the preferred method of repair. When considering open bypass or interposition grafting distal to the clavicle, key planning elements include (1) determination of optimal inflow and outflow, (2) conduit (almost exclusively autogenous vein), and (3) assessment of distal compartment pressures and potential need for fascial release. Commonly, more vasospasm is engendered by injury and surgical manipulation in the upper extremity arteries as compared to those of the lower extremities, and accommodations may need to be made to ensure graft and bypass patency in this context.
■ Branch vessel injuries and aneurysms, particularly those arising from the axillary and brachial arteries, are best treated with ligation and excision.14 Preoperative planning of these procedures involves selection of an appropriate exposure through the muscles of the upper extremity that will facilitate rapid recovery and minimize risk of disability in a usually young and active patient cohort.
■ The relatively superficial location of the brachial artery in the antecubital fossa increases its vulnerability to traumatic and iatrogenic injury.15 Most brachial injuries are associated with penetrating trauma; however, blunt injuries also occur, particularly in the distal brachial artery, following posterior elbow dislocations and supracondylar fractures (the latter more commonly in children).16 In these situations, key elements for repair will include inspection of injured arterial segment on preoperative imaging for possible intimal disruption, short segment thrombosis, or thrombosis extending distally into the forearm.
■ An increasing number of cardiac catheterizations and coronary interventions are performed via radial or brachial access.17 Cannulation site complications, including thrombosis or pseudoaneurysm formation, often necessitate operative repair.17,18 For these patients, preoperative planning will include identifying the extent of injury, options for graft conduit (smaller diameter vein), and alternative management options including arterial ligation in extenuating circumstances.
■ The ulnar artery at the wrist is the dominant hand artery in the majority of patients. Achieving or maintaining sufficient arterial outflow at the wrist is essential to the hemodynamic and clinical success of forearm revascularization procedures. The status of the radial and ulnar arteries at the wrist should be confirmed in the course of evaluating all patients for upper extremity revascularization options.
Operating Room Setup
■ The majority of upper extremity revascularization procedures are suited for a hybrid operating environment, or an OR equipped with a radiolucent, floating-point carbon fiber operating table and fluoroscopy radiation source and image intensification system, preferably equipped with digital subtraction angiography and last-image hold capabilities. When optimal x-ray penetration and resolution is not available, or in circumstances when diagnostic angiography alone is anticipated, less sophisticated portable imaging systems may suffice.
■ Elective and emergent upper extremity surgical revascularization procedures may be performed with either regional or general anesthesia. Considerations include the overall status of the patient, ability to tolerate the specific challenges associated with either anesthetic techniques, and the abilities of the anesthesiologist responsible for anesthetic management.
■ For the majority of upper extremity procedures, the operative limb is typically extended at 90 degrees. For optimal surgical exposure, we prefer arm positioning systems that move freely with the OR table rather than those with separate floor extensions. To avoid exacerbation of potential brachial plexus injuries in appropriate clinical settings, care should be taken to avoid hyperabduction and extension of the limb. The operative field should include, at a minimum, the ipsilateral axilla, chest, and neck, with the head rotated and extended to the contralateral side. A shoulder roll may be positioned under the ipsilateral shoulder to aid with neck and shoulder extension (FIG 1A). Alternatively, for optimal deltopectoral exposure of the axillary artery, the arm can be externally rotated and abducted at 30 degrees relative to the lateral chest.
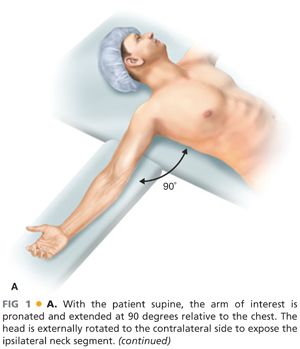
■ In situations where venous interposition conduit may be needed, a lower extremity should also be prepared into the surgical field to allow for greater or lesser saphenous vein harvest as indicated by the estimated diameter of the target artery. In the setting of extensive traumatic injuries, vein should be harvested from the least affected lower extremity.
TECHNIQUES
■ Systemic anticoagulation should be considered whenever major open or endovascular revascularizations are undertaken. Exceptions include profound systemic coagulopathy or concern related to concomitant risks such as occult intracranial hemorrhage. Sufficient intravenous unfractionated heparin should be administered to achieve an activated clotting time of more than 250 seconds.
■ Open surgical revascularization and repair techniques are described in the sections in the following text relative to upper extremity anatomic location.
■ The last section describes general endovascular techniques used for upper extremity interventions.
PROXIMAL AXILLARY ARTERY
First Step
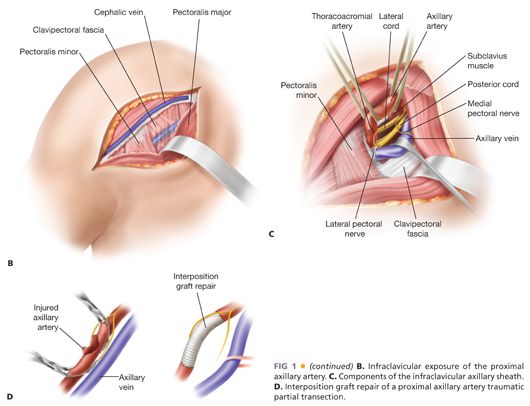
■ Exposure of the proximal (first) portion of the axillary artery is best achieved through an incision placed one fingerbreadth below the middle third of the clavicle (FIG 1B). Deep to the subcutaneous tissue, the pectoral fascia is opened longitudinally. The pectoralis major muscle is divided with a muscle-splitting incision. The underlying clavipectoral fascia is then sharply incised to expose the proximal axillary sheath. Additional exposure is aided by lateral retraction or division of the pectoralis minor muscle.
Second Step
■ Fine dissection should be used to expose and control the axillary artery deep to the clavipectoral fascia. Careful dissection and retraction minimizes injury to the cords of the brachial plexus surrounding the artery. The lateral pectoral nerve and proximal cephalic vein are also prone to injury during dissection or traction from misplaced self-retaining retraction devices.
Third Step
■ The axillary vein lies anterior and caudal to the artery within the axillary sheath. Mobility of the vein is achieved with gentle dissection, ligation of associated venous tributaries, and mild caudal retraction with a circumferential vessel loop or small handheld retractor (FIG 1C).
■ In smaller patients, division of the thoracoacromial artery and vein may be required to facilitate proximal axillary exposure. Once again, injury to the lateral pectoral nerve is avoided by gentle, deliberate dissection.
Fourth Step
■ Once circumferential dissection and exposure of the proximal axillary artery is complete, the artery is optimally controlled with silastic vessel loops (FIG 1C).
■ Familiar anatomic relationships may be less recognizable during redo or complex exposures, or in the setting of traumatic injuries, ongoing extravasation, and hematoma formation. The risk of associated brachial plexopathy is heightened in these situations. Extending exposure through the deltopectoral groove may help delineate otherwise indistinct tissue planes. Repositioning the arm throughout the range of available abduction may also reduce position-related anatomic distortion.
Fifth Step
■ For axillofemoral bypass grafting, the first or most proximal axillary segment is chosen for anastomotic access to minimize the risk of traction and potential graft disruption from shoulder and arm movement. Locating the anastomosis as proximate to the clavicle as possible optimizes long-term performance and durability. Based on the patient’s body habitus and planned graft configuration (uni- or bifemoral), an appropriately sized (6, 8, or 10 mm) externally supported expanded polytetrafluoroethylene (ePTFE) is employed. For most patients, in most situations, an 8-mm diameter, removable ring graft is optimal. The axillary arteriotomy is always created proximal to the overlying pectoralis minor muscle, which is itself usually divided to further minimize undue traction on the graft.
■ When considering direct ipsilateral axillo–axillo or axillobrachial bypass grafting, conduit choice depends on surgical context. In contaminated fields (with open penetrating or avulsion injuries of the axillary artery), vein is preferred and is sourced from the long or short saphenous or superficial femoral veins in the (least involved) lower extremity, or contralateral arm vein. For elective revascularization procedures, depending on the age of the patient, bypass length, target artery diameter, and indications for reconstruction, ePTFE or knitted polyester prosthetic grafts may provide acceptable alternatives. However, in nearly all situations requiring upper extremity bypass at or distal to the clavicle, autogenous vein is optimal and highly preferred.
■ For ipsilateral revascularization, graft is tunneled parallel to the existing axillary artery beneath the pectoralis major and minor muscles to the anterior axillary line.
■ For upper extremity revascularization procedures, tunneling is extended distally through subcutaneous planes along the arm and forearm as necessary to reach the target artery. In the case of axillofemoral bypass grafting, the ePTFE graft tunnel is created retrograde, extending from the femoral incision superiorly to the exposed axillary artery, with care being taken to position the graft anterior to the anterior superior iliac crest and advanced upward along the anterior axillary line. The tunnel must not breach the abdominal fascia or thoracic cavity. With a tunneling device of sufficient length, a counterincision is not usually necessary to reach the axillary artery. At the inferior border of the pectoralis muscle, the tunnel transitions to a subfascial plane extending below the pectoralis major muscle to reach the exposed axillary artery medial to its intersection with the pectoralis minor muscle. Use of a purpose-specific tunneling device for this maneuver will not only obviate the need for a counterincision but also minimize risks of kinking, twisting, or graft compression.
■ The conduit should be beveled appropriately for end-to-side anastomoses at both ends. At the axillary anastomosis, slightly more graft length redundancy is needed to prevent excessive traction on the anastomosis and late graft or arterial injury. The use of stretch polytetrafluoroethylene (PTFE) is also preferred for this reason.
Sixth Step
■ Traumatized, thrombosed, or aneurysmal proximal axillary artery segments may be transected or resected as necessary, reconstituted by interposition grafting with venous or prosthetic conduit (FIG 1D).
■ For interposition grafting, the damaged or diseased arterial segment is fully transected and removed. The lumen within the proximal and distal arterial segments should be inspected for trauma, dissection, or thrombus formation. In the case of the distal artery, flushing with heparinized saline may help confirm patency and sufficient runoff. Retrograde flushing of diseased or damaged brachial, axillary, or subclavian arteries is not recommended given the potential risk for vertebral artery embolization of residual luminal detritus and subsequent central nervous system (CNS) infarction or injury.
■ When uncertainty exists regarding the extent of axillary injury, further exposure may be necessary to ensure success. In rare and extenuating circumstances, clavicular resection and replacement (or removal) may be required for satisfactory arterial exposure. Similarly, when fragmented or chronically infected, the clavicle should be removed as necessary to optimize long-term graft patency and limb viability.
■ For upper extremity arterial reconstruction, once exposure is complete and the appropriate tunnel is created, the appropriate conduit is selected for use and prepared for interposition grafting (FIG 1D). The conduit should be fashioned to an appropriate length to avoid potential kinking during future arm motions. Proximal and distal anastomoses are performed end-to-end or end-to-side depending on the respective diameters of the inflow and outflow segments.
Seventh Step
■ Catheter embolectomy should be performed as necessary, often using over-the-wire, image-guided techniques, to remove luminal thrombus from inflow or outflow arterial segments as necessary. Care should be taken with proximally directed embolectomy to avoid dislodging clot fragments into the vertebral artery.
■ To minimize iatrogenic injury from embolectomy catheters, proximal embolectomy is best initiated at the level of the axillary rather than brachial artery. Attempting antegrade embolectomy proximally from an antecubital brachial access incision risks traumatic injury to the axillary or brachial artery at the origin of the deep brachial artery, where the diameter of the brachial artery decreases significantly, just distal to the axillary fossa.
■ Appropriately sized Fogarty thrombectomy catheters for upper extremity embolectomy include sizes 2 through 5 Fr, depending on the diameter of the artery being instrumented and the technique (antegrade or retrograde) being employed.
MID-DISTAL AXILLARY ARTERY
First Step
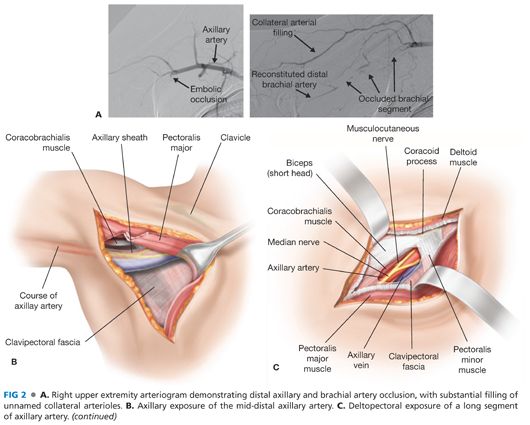
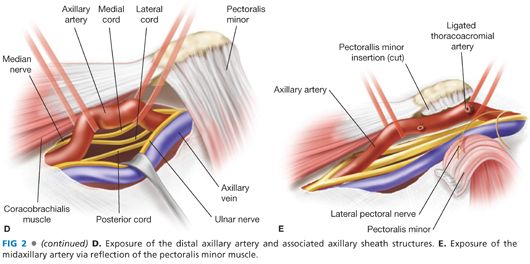
■ The mid-distal axillary artery may be exposed via an axillary or deltopectoral incision. For distal exposure, the incision is extended through the posterolateral border of the pectoralis major muscle to allow for partial mobilization and medial retraction of that muscle (FIG 2A). In the superior aspect of the surgical incision, the coracobrachialis muscle will be visualized at 90 degrees relative to the medially retracted pectoralis major muscle. Within this angle, gentle blunt dissection is applied to identify the axillary sheath running along the inferoposterior border of the coracobrachialis muscle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


